Spike S1 RBD (B.1.351, Beta Variant) (SARS-CoV-2): ACE2 Inhibitor Screening Colorimetric Assay Kit
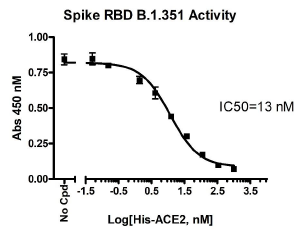
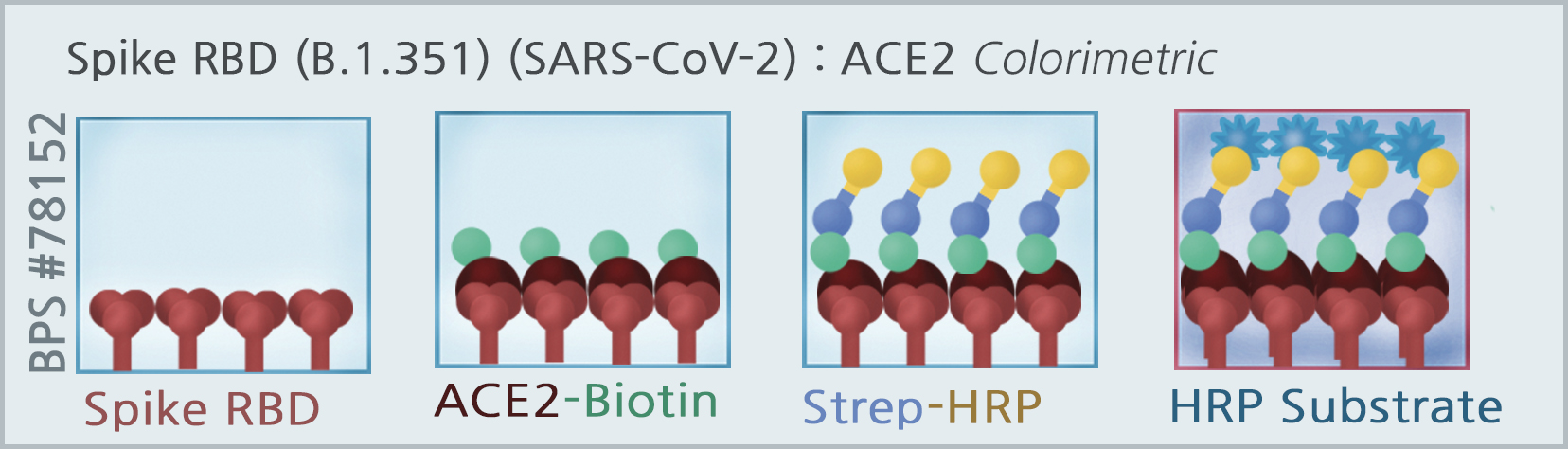
The Spike S1 RBD-B.1.351 (SARS-CoV-2): ACE2 Inhibitor Screening Colorimetric Assay Kit is designed for screening and profiling inhibitors of the interaction of ACE2 with the RBD region of the B.1.351 Variant of the SARS-CoV-2 Spike S1 protein. The key to this kit is the high sensitivity of detection of ACE2-Biotin protein by Streptavidin-HRP. Only a few simple steps on a microtiter plate are required for the assay. First, Spike S1 RBD-B.1.351 is coated on a 96-well transparent plate. Next, ACE2-Biotin is incubated with Spike S1 RBD-B.1.351 on the plate. Finally, the plate is treated with streptavidin-HRP followed by addition of an HRP substrate to produce color, which can then be measured using a UV/Vis spectrophotometer microplate reader.
Assay Principle
PBS (Phosphate buffered saline)
1N HCl (aqueous)
Rotating or rocker platform
UV/Vis spectrophotometer microplate reader capable of reading absorbance at 450 nm
|
Catalog |
Name |
Amount |
Storage |
|
5 µg |
-80°C |
||
|
5 µg |
-80°C |
||
|
15 µl |
+4°C |
||
|
50 ml |
-20°C |
||
|
50 ml |
+4°C |
||
|
|
Colorimetric HRP substrate |
10 ml |
+4°C |
|
|
Transparent 96-well white microplate |
1 |
Room Temp |
The pandemic coronavirus disease 2019 (COVID-19) is caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). As a first step of the viral replication strategy, the virus attaches to the host cell surface before entering the cell. The Receptor Binding Domain (RBD) of Spike protein recognizes and attaches to the Angiotensin-Converting Enzyme 2 (ACE2) receptor found on the surface of type I and II pneumocytes, endothelial cells, and ciliated bronchial epithelial cells. It has been widely suggested that active as well as passive immunizations targeting the interaction between the Spike protein of SARS-CoV-2 and human ACE2 offer promising protection against the viral infection.
A variant strain of SARS-COV-2, identified as B.1.351, was first identified in the fall of 2020 in the Republic of South Africa. This South African variant, also known as 501Y.V2, has many mutations which may lead to higher transmissibility and infectivity. In particular, the B.1.351 variant contains three mutations within the RBD region of the Spike S1 protein: K417N, E484K, and N501Y. Investigating the effect of mutations on viral replication and pathogenesis will be critical for developing effective strategies for vaccines and antibody therapies against COVID-19.
1. Wang P. et al., Increased Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7 to Antibody Neutralization. bioRxiv 2021 Jan 26; 2021.01.25.428137
2. Shen X., et al., SARS-CoV-2 Variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral Spike vaccines. bioRxiv. 2021 Jan 29; 2021.01.27.428516
3. Hoffman M. et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181:1-10