Protein Binding Studies / BLI Detection Services
Bio-Layer Interferometry (BLI) is a powerful analytical technique for studying biomolecular interactions. BLI measures changes in interference patterns of light reflected from a biosensor tip, which is typically coated with a layer of molecules relevant to the study. As biomolecules bind to or dissociate from the biosensor surface, the interference patterns change, providing real-time data on the kinetics and affinity of these interactions.
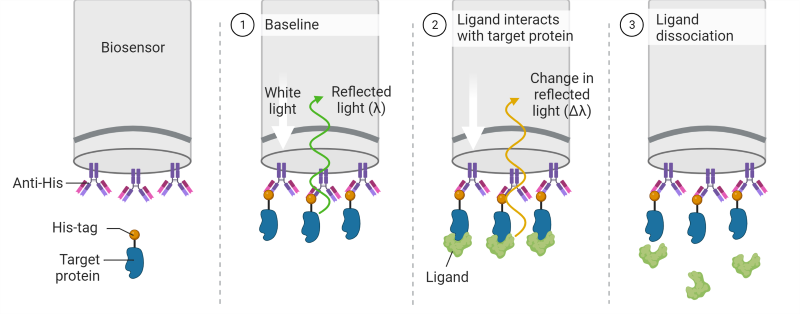
One key benefit of BLI is that it provides a label-free and real-time monitoring of biomolecular interactions. Unlike traditional methods that may require labeling with fluorescent or radioactive tags, BLI allows researchers to study interactions in their natural state. This not only simplifies experimental procedures but also avoids potential alterations in the behavior of the molecules under investigation. Furthermore, BLI provides versatility in studying various types of biomolecular interactions, including protein-protein, protein-small molecule, and protein-nucleic acid interactions. Its sensitivity and speed make it a valuable tool for characterizing binding kinetics, affinity constants, and concentration-dependent effects, providing crucial insights into the mechanisms underlying biological processes.
Total Solutions for BLI
We offer start to finish services to deliver your protein binding data quickly. Start by selecting from our extensive library of off-the-shelf proteins, your proteins, or custom manufactured proteins. We will provide:
- Protein immobilization
- Binding affinity measurements using multiple experimental points
- Data fitting and reporting
- A final report outlining all data, methods, and results
Example Data Sets
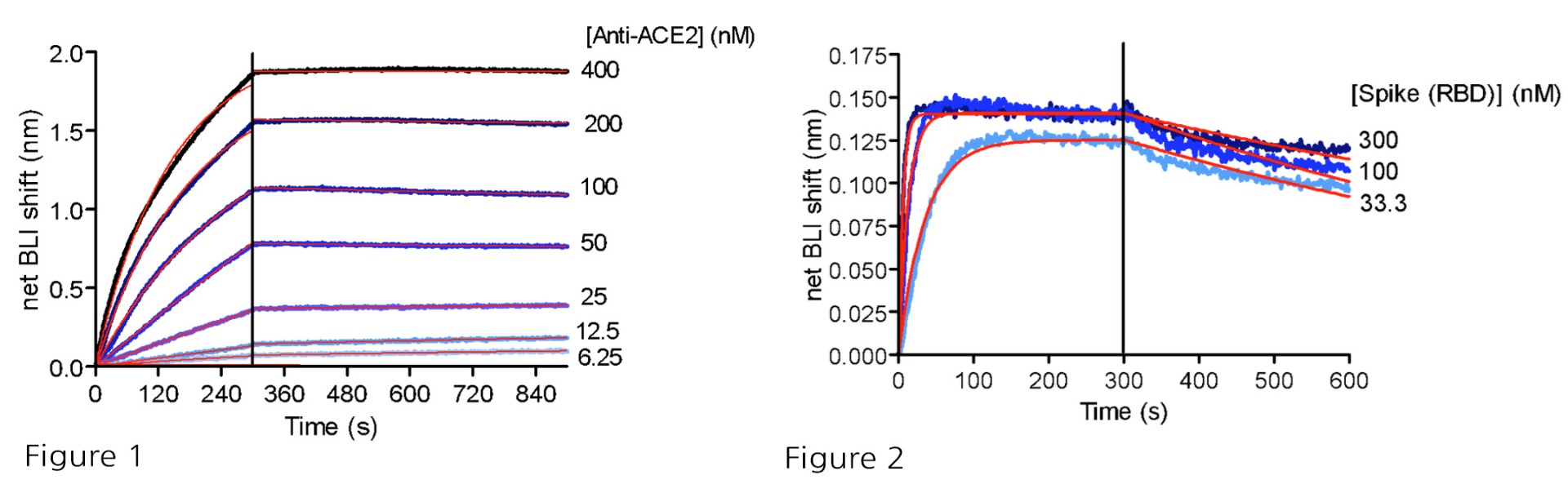
Figure 1: BLI binding analysis of human anti-ACE2 antibody to immobilized ACE2-His (#11003) via anti-His probes. Kd = 144 nM.
Figure 2: BLI binding analysis of SARS-CoV-2 Spike (RBD) to immobilized ACE2-His (#11003) via anti-His probes. Kd = 1.2 nM.
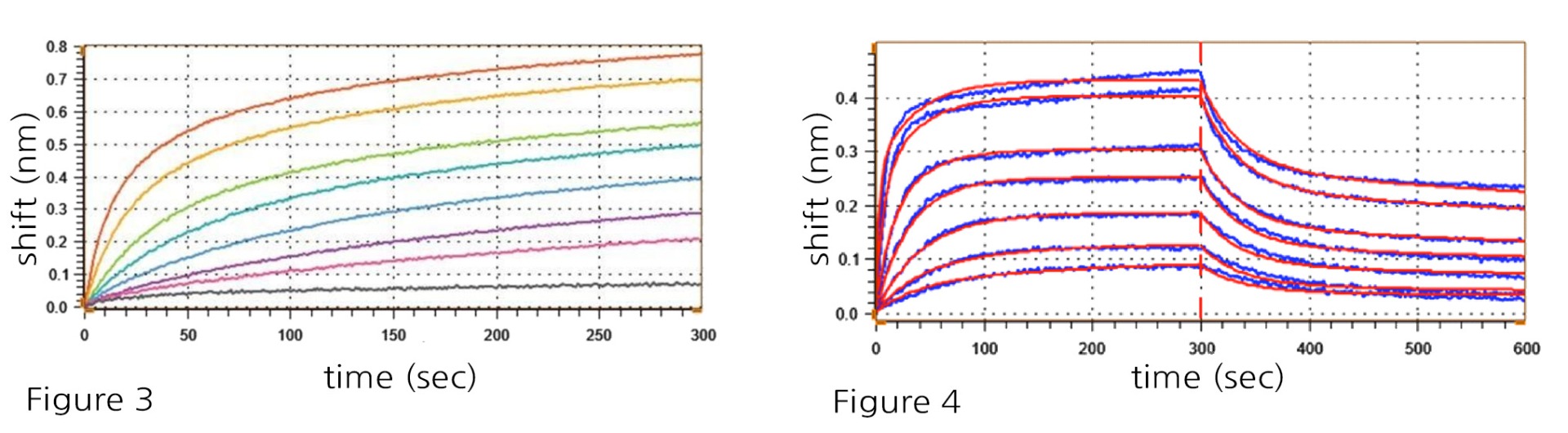
Figure 3: PD-1-His (#71198) immobilization with anti-His probes using a 2-fold serial dilution range from 400 to 6.25 nM.
Figure 4: Association and dissociation kinetics of PD-1-His (#71198) immobilized at 100 nM against PD-L1 (#71104) titrated from 400 to 6.25 nM in a 2-fold serial dilution, reveals the following results: koff = 5.21 ± 1.03 x 10-4 s-1: kon = 7.44 ± 0.05 x
10-5 M-1 s-1, Kd = 0.7 nM
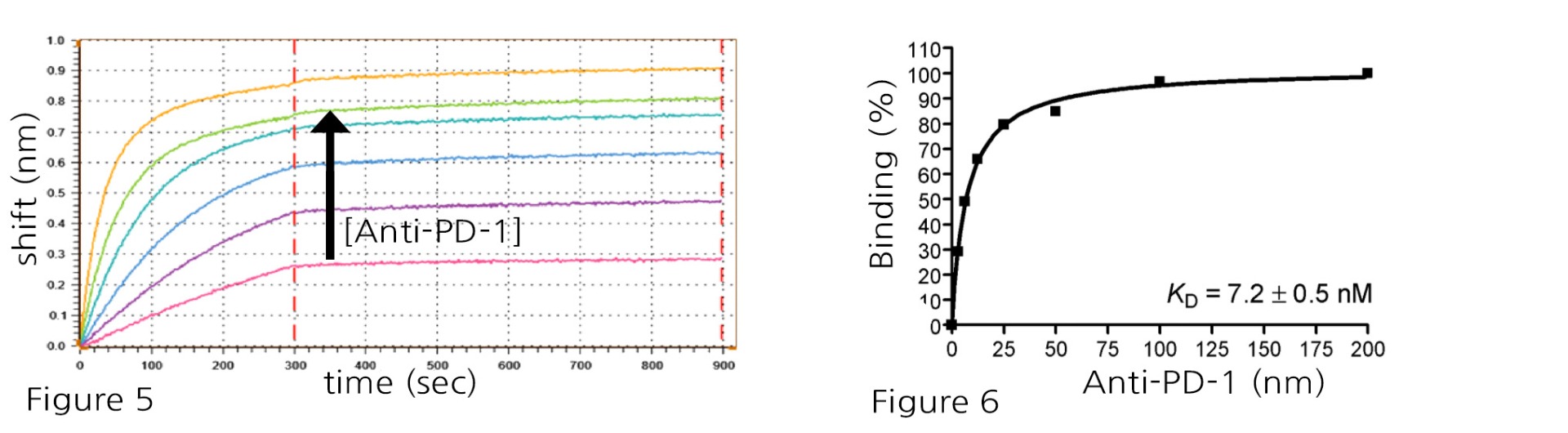
Figure 5 and Figure 6: PD-1-His:Anti-PD-1 antibody binding using 100 nM immobilized PD-1-His (#71198) against Anti-PD-1 (#71120) titrated from 100 to 3.125 nM in a 2-fold serial dilution, revealed the following: Anti-PD-1 binds to immobilized PD-1 with high affinity, showing a very slow dissociation (unable to obtain accurate koff), Kd = 7.2 ± 0.5 nM at steady state.

