Custom CAR-T Cell Development
CAR-T cell therapy is a revolutionary cancer treatment that involves genetically engineering immune cells, using either the patient’s own cells or allogeneic cells, to recognize and attack cancer cells using a Chimeric Antigen Receptor (CAR). This therapy has shown remarkable success in treating some types of blood cancers, such as leukemia and lymphoma, even in patients who have not responded to other treatments. While CAR-T cell therapies are a breakthrough in the field of cancer treatment, more development is required to improve overall safety and efficacy against solid tumors, as well as use in other disease areas.
To advance your CAR-T cell research, BPS Bioscience offers full service custom CAR-T development, from CAR design through to cellular functional validation and expansion. Our expertise in lentivirus production and cellular engineering will get you reliable results fast.
A Milestone-Measured Process from Concept to CAR-T
The development of custom CAR-T cells is a complex process that requires a stepwise workflow, implemented in stages:

Researcher provides Ab sequence against antigen

Engineering and validation of ScFv for specificity and affinity
CAR Lentivirus production and initial validation

Researcher provides Ab sequence against antigen

Engineering and validation of ScFv for specificity and affinity

CAR Lentivirus production and initial validation
- Design and construct lentivector for scFv-CAR of different varieties including mono-scFv, dual scFv, and tandem scFv CARs.
- Transfect lentivector and packaging plasmids in 293T cells to make high titer, concentrated virus.
- Validate CAR construct by transducing into the NFAT reporter Jurkat cell line to check for expression of CAR by flow cytometry and for functional activity by reporter assay.
T cell preparation and transduction.
Functional validation of CAR-T cells
CAR-T cell expansion and optimization

T cell preparation and transduction.

Functional validation of CAR-T cells

CAR-T cell expansion and optimization
- Isolate, activate and expand primary T cells (from healthy donors).
- Transduce T cells and check the expression of CAR by flow cytometry.
- Establish target cells stably expressing antigen of interest in a luciferase reporter CHO cell line.
- Measure IFNγ production by CAR-T cells in co-culture assays using BPS Bioscience’s IFNγ ELISA kit.
- Assess cytotoxic cell killing using co-culture of target cells and effector CAR-T cells.
Customization to Meet Specific CAR-T Aims
- Compound and antibody screening using CAR-T cells or CAR/NFAT reporter Jurkat cells
- Intracellular costimulatory and activation domain comparisons
- CAR-T signaling in the tumor microenvironment
- Screening CAR-T donor variations
- Evaluate Treg and T memory cells in the CAR-T setting
Functional Validation
Expression Confirmation
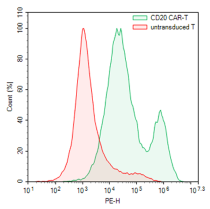
Flow cytometry of CD4+CD8+ T cells transduced with anti-CD20 CAR Lentivirus using biotinylated Protein-L and PE-Streptavidin.
IFNγ Secretion
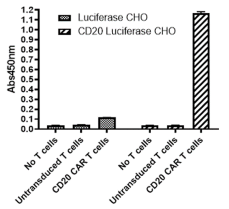
Quantitation of IFNγ secretion by anti-CD20 CAR-T or control cells co-cultured with either Luciferase CHO or CD20 Luciferase CHO cells, measured using BPS Bioscience IFNγ ELISA Detection Kit (#79777).
Cell Killing
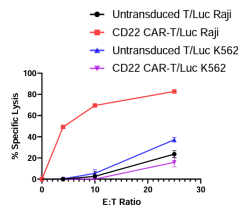
Determination of anti-CD22 CD4+CD8+ CAR-T cell specific killing of Luciferase Raji cells in a co-culture assay. The assay was performed in parallel with untransduced T cells and Luciferase K562 cells as negative controls.
Expression Confirmation

Flow cytometry of CD4+CD8+ T cells transduced with anti-CD20 CAR Lentivirus using biotinylated Protein-L and PE-Streptavidin.
IFNγ Secretion

Quantitation of IFNγ secretion by anti-CD20 CAR-T or control cells co-cultured with either Luciferase CHO or CD20 Luciferase CHO cells, measured using BPS Bioscience IFNγ ELISA Detection Kit (#79777).
Cell Killing

Determination of anti-CD22 CD4+CD8+ CAR-T cell specific killing of Luciferase Raji cells in a co-culture assay. The assay was performed in parallel with untransduced T cells and Luciferase K562 cells as negative controls.
Quote Request
Inquiries
│Related Services
│Related Products
- TCellM™ Optimized Media
- Cell Lines & Primary Cells
- Cell Media & Luciferase Reagents
- Cell Isolation Kits

