Lentiviruses
Lentiviral transduction is a powerful tool for introducing genes into cells and is used extensively for cellular engineering. It has recently made its way to the clinic with ex-vivo cell therapy applications. Lentiviruses (LV) have also been used extensively for the modeling of SARS-CoV-2 viral infection of human cells.
Lentiviral transduction is a powerful tool for introducing genes into cells and is used extensively for cellular engineering. It has recently made its way to the clinic with ex-vivo cell therapy applications. Lentiviruses (LV) have also been used extensively for the modeling of SARS-CoV-2 viral infection of human cells.
Today’s methods of LV production ensure they are very safe. Thus, a biosafety level 2 facility is sufficient for using BPS Bioscience’s lentiviruses. Our replication-incompetent viral particles, produced from packaging cells, contain minimal genetic information that includes the gene of interest but does not include the viral packaging genes. Therefore, cells infected with our viruses cannot produce new viral particles. All BPS Bioscience LV products are supplied as ready-to-use viral particles that meet specifications in terms of titer and size. Exact titers are indicated with each lot.
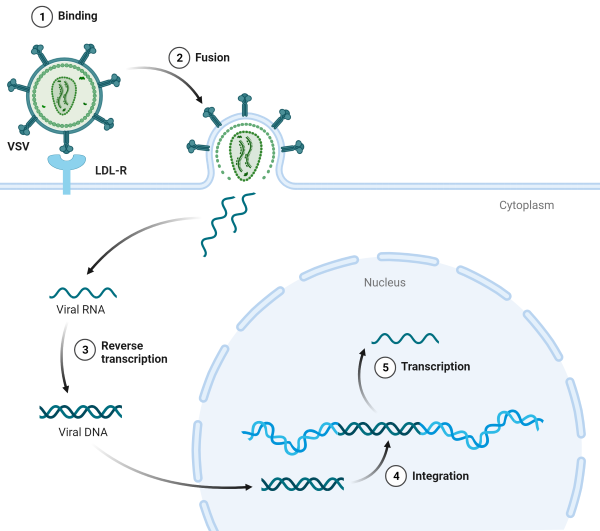
LVs are enveloped viruses that enter cells by binding to a particular cell surface protein, followed by membrane fusion, which pours into the cell’s cytoplasm the virus RNA containing its genetic information. This single-stranded viral RNA is turned into DNA by reverse transcription. The viral DNA enters the nucleus and is integrated into the host cell genome by a viral integrase. The most used LV is based on RNA virus HIV (human immunodeficiency virus) that infects human lymphocytes through the specific interaction of its envelope protein gp120 with the T cell CD4 receptor. In biotechnology applications, gp120 has been replaced by another envelope glycoprotein present on the surface of most mammalian cells to expand viral tropism.
1) LV for Gene Transfer
Typically, LVs used for gene transfer contain VSV-G (Vesicular stomatitis virus G protein) as their envelope glycoprotein, which binds to the human LDL receptor (low-density lipoprotein receptor) expressed on most cells, making these virus particles especially useful to target a wide range of cell types.
These VSV-decorated viral particles bind to cell surface LDLR and fuse with the plasma membrane. The viral RNA is transferred into the cells to be processed by the cellular machinery, The resulting DNA is eventually integrated into the cell’s genome, resulting in stable transcription and expression of the protein.
LV Advantages
- Versatility: LV infects both actively-dividing and non-dividing cells and is ideal for transducing primary cells or stem cells, which can be resistant to other methods of transfection
- Stable integration into the genome which leads to stable expression of the transgene
- Large cargo capacity: the size of the inserted DNA can be up to 10 kb
- High transduction efficiencies (up to 100% in some cell lines)
- Low cellular toxicity
2) Spike-pseudotyped LV for Cellular Infection Modeling
These viral particles provide a safe platform for studying SARS-CoV-2 viral infection in cellular systems. They feature the coronavirus spike protein on their surface instead of gp120 or VSV-G and infect human cells through the binding of Spike to membrane receptor ACE2 (angiotensin-converting enzyme 2). BPS Bioscience’s Spike pseudo-viruses transduce a reporter gene to provide quantitative measures of infection efficacy.
Their unique characteristics enable the study of spike/ACE2 interactions and provide opportunities for various applications, including the exploration of therapeutic approaches, compound library screening, determination of compound IC50, and more.
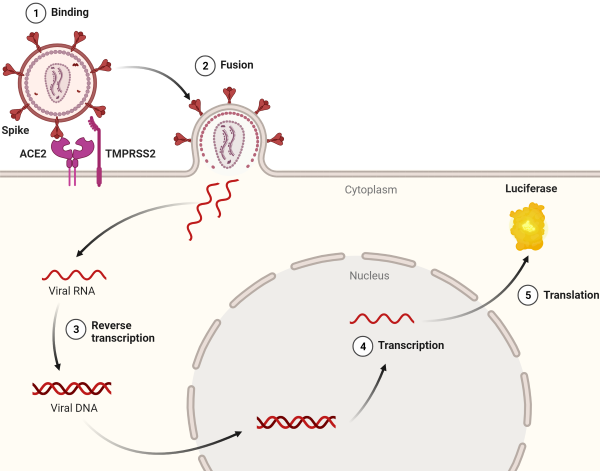
Cells expressing high levels of ACE2 are ideal models for studying this interaction. ACE2-overexpressing cell lines such as ACE2-HeLa, ACE2-CHO, and ACE2-HEK293 cells have validated ACE2 overexpression. Vero E6 cells, which endogenously express ACE2, are also commonly used in coronavirus studies. We recommend employing TMPRSS2-Vero E6 cells since the protease facilitates Spike cleavage, promoting membrane fusion and virus entry into the cells.
LV Products

Bald Lentiviruses are negative control viral particles in which the envelope protein has been removed entirely. Therefore, bald LV cannot interact with host cells in a receptor-specific fashion. Bald lentiviruses serve as negative controls (or blank) in transduction experiments. They also allow to estimate the proportion of transduction resulting from non-specific spinoculation as opposed to transduction mediated by specific receptor binding when modeling viral infection or when optimizing transduction protocols.

Off the-shelf CAR and TCR Lentiviruses facilitate the expression of validated CAR (Chimeric Antigen Receptor) or TCR (T Cell Receptor) constructs targeting specific targets of interest to use as positive controls during the development of CAR or TCR-expressing cells for adoptive cell therapy.

Knocking out the expression of a gene of interest is particularly valuable in target validation studies or to examine the biological function of the gene. The CRISPR/Cas9 Knock-out Lentiviruses transduce Streptococcus pyogenes Cas9, along with 5 single-guide RNAs (sgRNAs) targeting a gene of interest. They can be used for the generation of stable knock-out cell lines following cell selection.

Cell Signaling Pathway lentiviruses transduce luminescent (firefly luciferase, Renilla luciferase) or fluorescent (RFP, YFP, eGFP) reporter proteins under specific promoters. Expression of the reporter protein, therefore, is induced in response to the activation of specific transcription factors downstream of a pathway of interest.

Spike-pseudoviruses model Spike-mediated infection of human cells by binding to ACE2. Spike pseudoviruses corresponding to SARS-CoV-1 and SARS-Co-V2 strains are available, as well as many Spike mutants from identified SARS-CoV-2 variants.

Bald Lentiviruses are negative control viral particles in which the envelope protein has been removed entirely. Therefore, bald LV cannot interact with host cells in a receptor-specific fashion. Bald lentiviruses serve as negative controls (or blank) in transduction experiments. They also allow to estimate the proportion of transduction resulting from non-specific spinoculation as opposed to transduction mediated by specific receptor binding when modeling viral infection or when optimizing transduction protocols.

Off the-shelf CAR and TCR Lentiviruses facilitate the expression of validated CAR (Chimeric Antigen Receptor) or TCR (T Cell Receptor) constructs targeting specific targets of interest to use as positive controls during the development of CAR or TCR-expressing cells for adoptive cell therapy.

Knocking out the expression of a gene of interest is particularly valuable in target validation studies or to examine the biological function of the gene. The CRISPR/Cas9 Knock-out Lentiviruses transduce Streptococcus pyogenes Cas9, along with 5 single-guide RNAs (sgRNAs) targeting a gene of interest. They can be used for the generation of stable knock-out cell lines following cell selection.

Cell Signaling Pathway lentiviruses transduce luminescent (firefly luciferase, Renilla luciferase) or fluorescent (RFP, YFP, eGFP) reporter proteins under specific promoters. Expression of the reporter protein, therefore, is induced in response to the activation of specific transcription factors downstream of a pathway of interest.

Spike-pseudoviruses model Spike-mediated infection of human cells by binding to ACE2. Spike pseudoviruses corresponding to SARS-CoV-1 and SARS-Co-V2 strains are available, as well as many Spike mutants from identified SARS-CoV-2 variants.

