The Power of Lentiviruses in Cell Engineering
Introduction
The ability to manipulate nucleic acids has been a key discovery that has driven research, and ultimately clinical applications, forward. It opened the door to study the impact of mutations in cellular models, dissect pathways, generate recombinant proteins and monoclonal antibodies, and is the basis of gene and cell therapy, continuing to lead to new discoveries and cure patients every day. The methods used to create those manipulations differ in efficiency, permanence of expression, safety, and scalability. Here we will focus on lentiviruses, one of the most widely used tools for gene delivery.
The ability to manipulate nucleic acids has been a key discovery that has driven research, and ultimately clinical applications, forward. It opened the door to study the impact of mutations in cellular models, dissect pathways, generate recombinant proteins and monoclonal antibodies, and is the basis of gene and cell therapy, continuing to lead to new discoveries and cure patients every day. The methods used to create those manipulations differ in efficiency, permanence of expression, safety, and scalability. Here we will focus on lentiviruses, one of the most widely used tools for gene delivery.
Lentiviruses as engineering tools
During initial in vitro research studies investigators can rely on techniques such as electroporation and transfection to introduce genes into their cultured cells. This is however not always feasible when studying primary cell lines, as these cells are usually hard to transfect, can have cellular responses and their plasma membrane tends not to be permeable to DNA-lipid complexes. Furthermore, electroporation and lipid-mediated transfection are not applicable to more complex pre-clinical studies, such as in vivo analysis.
Lentivirus (LV) transduction has a few advantages over other electroporation or transfection methods, particularly in terms of efficiency, stability, cell type versatility, and cargo capacity. Thanks to its high rate of infection, lentivirus transduction tends to be more efficient, meaning that it can introduce genetic material into a higher percentage of cells. It is also less toxic to the cells and allows for larger cargo capacity (up to 10 Kb). Most importantly, lentiviruses can integrate into the genome, resulting in stable and long-lasting expression of the introduced gene, while other methods typically result in transient expression with integration being a very rare event.
Most of the highly impactful scientific tools have been developed by observing and mimicking what already exists in nature, and further enhancing it. This has been the case with lentiviruses used as gene delivery tools. Lentiviruses are retroviruses, from the Retroviridae family, since their genome is RNA based and requires reverse transcription. They contain only eight genes: gag, pol, rev, tat, nef, vif, vpr, vpu and env. The gag gene encodes matrix (MA), capsid (CA) and nucleoproteins (NC), while pol encodes the viral enzymes (reverse transcriptase (RT), protease (PR) and integrase (IN)). Their host cell cycle starts with direct membrane fusion or receptor-mediated endocytosis. Upon entry into the cells, the matrix and capsid structures denaturate, revealing the viral RNA, which is converted into double stranded DNA, the viral DNA. The viral DNA translocates to the nucleus via an active transport mechanism that uses importins. Since viral DNA can enter through an intact nuclear membrane, lentiviruses can be efficacious in non-dividing or terminally differentiated cells that do not undergo mitosis, a significant advantage compared to other viruses. The viral DNA then integrates into the host cell’s genome and utilizes the host’s machinery for transcription and translation. Finally, new viruses are assembled and released (1).
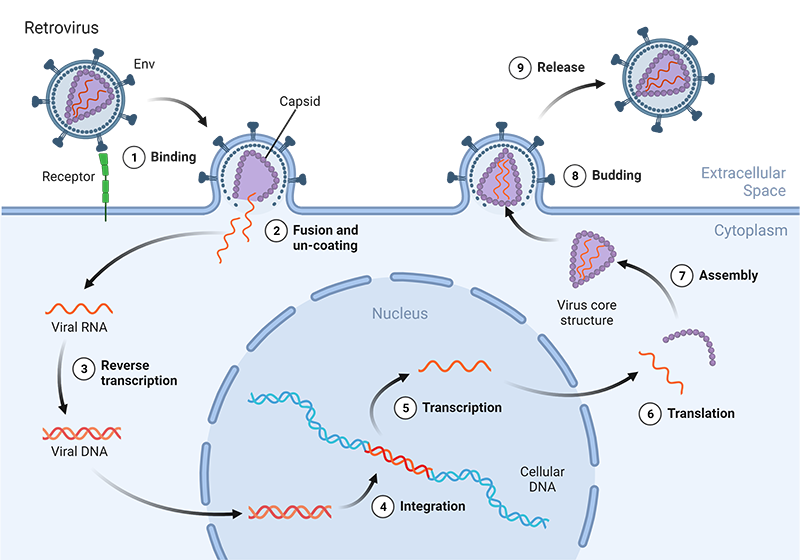
Figure 1: Illustration of the HIV lentivirus life cycle. Viruses can infect host cells via receptor-mediated endocytosis or direct membrane fusion. The viral envelope is dissembled, and their RNA is converted into DNA. This viral DNA penetrates the nucleus where it integrates into the host cell’s genome. Using the host cell’s machinery viral proteins are produced and new viral particles assembled and released.
The most used lentiviruses in research are HIV-based, pseudotyped lentiviruses. HIV lentiviruses exhibit efficient gene transduction leading to robust expression of the desired gene when paired with a strong promoter. Initial lentivirus systems included a significant portion of the HIV (human immunodeficiency virus) genome divided into two plasmids, and included genes like vif, vpr, vpu and nef. These genes are not essential for viral replication, so they were removed when a second-generation lentivirus system was developed, with each generation aiming at higher efficiency and safety. Third generation lentiviruses had the tat gene removed. The fourth generation was created by dividing the minimal essential genes into four plasmids, creating not only a higher level of safety but also “space” in the genome for larger transgenes. Additionally, deletions into the LTR (Long Terminal Repeat) created SIN (self-inactivation) lentiviruses, a major step towards preventing the chance of producing a full viral RNA and its activation.
Although most of the available lentivirus tools are based on HIV, it is worth mentioning that non-primate viruses have also been considered. EIAV (equine infections anemia virus) and FIV (feline immunodeficiency virus) are two of the types that have been explored. The glycoprotein gp120 present at the surface of the virus, which normally binds to CD4 on human T cells, has been replaced by the VSV-G protein (vesicular stomatitis virus G protein) which binds to the LDL receptor (low-density lipoprotein receptor) present on the surface of most mammalian cells, expanding their cell tropism. The ability to extend lentivirus tropism by creating various pseudotypes, in which the envelop glycoproteins are engineered from several species, has also contributed to even wider applications. For example, using the envelope proteins from Mokola virus (MK), Ross River virus (RRV) and Rabies virus (RV) allowed efficient transduction of neuronal cells (3). Often the choice of a gene promoter fell on CMV, as it results in robust expression in most cell types. However, CMV promoters are not really useful for resting cells, so there is now a preference for constitutively active cellular promoters such as elongation factor 1-α (1). This versatility makes them broadly applicable.
Some of the characteristics that have initially attracted researchers to use lentiviruses have also proved to be their downsides, particularly in the clinic. Serious concerns about retroviruses random genome integration were raised when patients with SCID-X1 (X- linked severe combined immunodeficiency) and ADA-SCID (adenosine deaminase deficiency) that had been treated with γ-retroviruses developed cancer, and provirus DNA was found inserted near protooncogenes. While we tend to consider that viral DNA integrates randomly in the host’s genome, that is however not strictly true, with lentiviruses favoring integration near sites of active chromatin. Lentiviruses, by nature, carry lower risks than γ-retroviruses, requiring a much larger dosage to get a similar oncogenic risk. However, they are not risk free. Thus, the field has been moving towards the use of non-integrating lentiviruses, where the integrase protein is mutated. These particles remain mostly episomal, avoiding the risk of oncogene activation.
Lentiviral Production
Lentiviral production is a multistep process that may seem straightforward but has several nuances that can severely impact titer and purity. It is often one of the most expensive steps in a cell therapy workflow, and a major roadblock, as high purity and titers, measured in number of viral particles capable of transducing cells such as TU (Transducing units), are required.
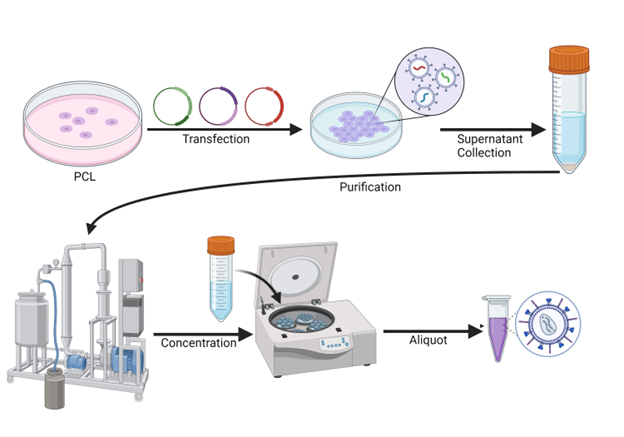
Figure 2: Lentiviral manufacturing workflow. Lentiviral vectors are delivered to a packaging cell line (PCL) via transfection, and viral particles are produced and released into the supernatant. The supernatant is then collected, purified, and concentrated to generate purified high titer solutions. These need to be dispensed into appropriate volumes for storage as lentiviruses are sensitive to freeze/thaw cycles.
Production of viral particles relies on using a host cell line to produce the viral components and to package them into particles. This cell line is designated as a packaging cell line (PCL) and should have no sequence homology to the vectors being used and no homologous endogenous viruses, to avoid any risk that replication-competent viruses are created. HEK293T cells are often the PCL of choice, as they are easy to transfect and manipulate and contain the SV40 large T antigen, which results in a better yield. To generate the volume and titers needed, particularly for in vivo applications, a large cell number is required, demanding large scale expansion in platforms such as cell stacks. Stacks can go up to 42 layers, being able to reach a production of 6 x 1011 TU (4). These are, however, hard to manipulate, take up space, and consume large volumes of media and serum, which are difficult to process in later stages. The use of HEK293 cells adapted to suspension culture, combined with bioreactors and serum-free media have expanded capabilities. For example, the use of an iCELLis 500 system can be equivalent to the use of 3000 HYPERFlasks (4). The use of cells in suspension brings the advantages of allowing easy scale up, not requiring dissociation steps, use of serum-free media, and higher cell densities.
Efforts to improve safety resulted in the viral genome being divided into several plasmids. Increased safety comes at the cost of decreased transfection efficiency, and additional effort into fine tuning the ratios and transgene sizes of the several plasmids were needed. For multi-plasmid transfection to be effective optimized cell confluency, transfection methods and DNA ratios are critical. Several transfection methods are available, which vary in cost and characteristics. Calcium phosphate (CaPO4) precipitation is a cheap method, but it is highly sensitive to pH and it can be cytotoxic, requiring serum and media change. The use of PEI (polyethylenimine) is an alternative that doesn’t require media change or seems to be impacted by the pH, while being as effective, but the DNA:PEI ratio has a very large impact on the efficiency. Lipid-based reagents are very efficient but become too expensive for large scale. A stably transfected packaging cell line would alleviate many of the costs and efforts into reproducing the exact conditions used in a successful manufacturing run. However, overexpression of proteins such as VSV-G, Rev, gal-pol is toxic to the cells, making the development of such stable cell lines quite challenging.
Over the next few days after transfection, cells produce viral particles, which are released into the cell culture media. It is critical to have an optimized cell density at transfection in order to maximize the number of cells producing viruses prior to cell senescence and death. For instance, when using adherent cells, one should keep in mind that reaching full confluency too soon may lead to cell detachment prior to optimal viral production. The viruses need to be purified to remove DNA contaminants, proteins, fragments of the PCL, serum, etc. as impurities can lead to low transduction efficiency and to inflammatory responses following administration to patients. Particularly when large transgenes are of interest, which can result in titers being 10-fold lower than with smaller transgenes, scale up and concentration steps are required to obtain workable titers. In a first step, clarification with filters of consecutive smaller size can be performed. The virus can then be concentrated by TFF (tangential flow filtration), which also removes serum and DNA fragments. This is followed by chromatography steps, such as anion exchange chromatography, and finally SEC (size exclusion chromatography) can be used to polish out impurities (3).
Viruses have a short half-life, requiring storage as stocks at -80°C in a cryoprotective solution, such as sucrose and magnesium. They are also sensitive to freeze/thaw cycles, so particular care is needed with stocks and creation of single use aliquots.
Overall, while the production of lentiviruses uses techniques commonly used in most laboratories, trial and error can lead to delays and escalating costs, severely impacting the feasibility of pre-clinical and clinical studies. Partnering with a company or laboratory that has invested in optimizing and understanding the impact of each step of the process on the final product is often a time and cost saving path followed by most.
Viral Titers
Lentiviral protocols for both in vitro and in vivo transduction are based on viral titers. Titers can vary greatly with the method used in its assessment. These methods can be functional and determine the actual number of viral particles capable of transducing cells and tend to be expressed in TU. They require serial titration and use of standards in cells and read outs such as flow cytometry or imaging. Other methods such as ELISA for viral proteins and PCR-based methods are alternatives that can provide rapid results but can result in an overestimation of titer by measuring “dead” particles. Even when using cell-based methods, viral titering often makes use of cells lines that are highly permissible and may not translate directly to harder to transduce cell lines, or to the conditions used in downstream applications. Factors such as media, volume and timing of transduction may also have an impact on the titers required. The viral dosage to be used links to the targeted cell type/organ, number of receptors on the cells, and it is thus recommended that in addition to using titers based on functional viral particles, small scale studies are done.
Lentiviral Applications as Cell Engineering Tools
The applications of lentiviruses are as many as the cellular genetic manipulations one can think of. In the clinical setup they provide the main tool for gene therapy, allowing long term and steady “state” dosing with a gene of interest, and are an accessory tool in cell therapy.
In gene therapy, lentiviruses deliver a missing gene or replace a non-functional one. For example, the eye is an immune-privileged organ and amenable to noninvasive surgery, and such properties made it a model organ for gene therapy. Several successful clinical trials targeting diseases such as Stargardt, Usher Syndrome 1B and wet AMD (age-related macular degeneration) are underway.
In cell therapy, lentiviruses are not the actual therapeutic tool but are nevertheless critical to deliver the desired transgenes or differentiation factors. Allogenic transplantation of HSC (hematopoietic stem cells) has been a treatment option for patients that require reconstitution of their hematopoietic lineage, but GvHD (graft versus host disease) remains a critical concern. The use of autologous HSC, where mutations that lead to the disease were corrected, has become an advanced option of treatment. The use of CD34+ HSC genetically manipulated with lentiviruses has been successful in the treatment of β-thalassemia, Wiskott-Aldrich syndrome (WAS), X-SCID and sickle cell disease (SCD). Lentiviruses are also being used to introduce tumor-specific TCRs (T-Cell Receptors), such as NY-ESO1 and MART-1, and CAR (chimeric antigen-receptors) into T cells. Clinical trials using anti-CD19 CAR T-cells demonstrated high efficacy, with durable response rates in patients with relapsed or refractory B-cell acute lymphoblastic leukemia (1).
Table 1: Examples of targets and lentiviral tools used in ongoing clinical trials (4, 5).
| Pathology | Gene | Lentiviral Vector |
|---|---|---|
| SCID-X1 | IL2RG | G2SCID LV |
| TYF-IL-2Rg self-inactivating LV (TYF-IL-2Rg) | ||
| LV VSV-G pseudotyped CL20-4i-EF1a-hyc-OPT | ||
| ADA-SCID | ADA | EFS-ADA LV |
| self-inactivating LV TYF-ADA | ||
| WAS | WAS | w1.6_hWASP_WPRE (VSVg) LV |
| Transfusion-dependent β-thalassemia | HBB | GLOBE LV |
| LV βA-T87Q-globin | ||
| SCD | HBB | LV βA-T87Q-globin |
| γ-globin LV | ||
| GLOBE1 LV Expressing the βAS3 Globin Gene | ||
| Lenti/G-βAS3-FB LV | ||
| MLD | ARSA | LV ARSA |
| X-ALD (X-linked adrenoleukodystrophy) | ABCD1 | SIN LV MNDprom-ABCD1 (Lenti-D) |
| autologous CD34+ cells transduced with SIN LV MNDprom-ABCD1 (Lenti-D) |
Some aspects related to the use of lentiviruses in the clinic still need to be considered, and potentially addressed. For instance, individuals who have received lentiviral vector-based gene therapies that made use of HIV backbones may test positive on some HIV testing platforms, depending upon the vector construction and detection reagents used. Differentiating these cases from natural HIV-1 infection is thus critical (1). Patients who currently receive treatment need to undergo repeated testing during the first-year post-treatment, and require subsequent follow ups as the long-term overall safety in terms of neurological, rheumatologic and autoimmune disorder development is still unclear, and there are still concerns with insertional mutagenesis.
The use of lentiviruses to introduce CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9 has attracted recent attention. Their application started with libraries of sgRNAs used for genome-wide screens. These genome-wide screens helped to identify genes essential for cell viability in cancer and pluripotent cells, genes involved in drug resistance and functionally relevant genes for disease development and progression and identify epigenome-modifier enzymes and chromatin modifiers. However, the use of lentiviruses to deliver CRISPR/Cas9 has unique challenges. Prolonged or excess expression of gRNA/Cas9 can result in off-target effects, making the use of integrase deficient lentiviruses a more appropriate path (3).
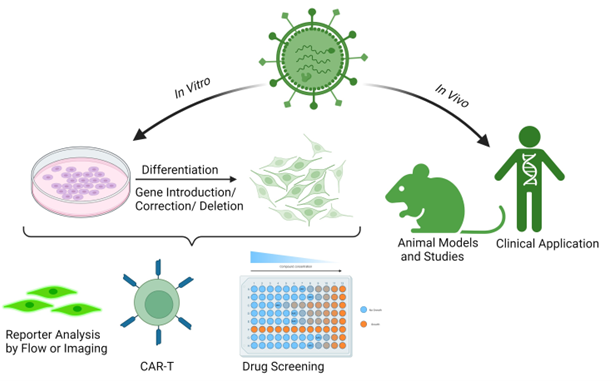
Figure 3: Generic lentiviral applications as cell engineering tools. Lentiviruses can be used for in vitro or in vivo cell delivery. In vitro delivery can provide cells with reporter genes, corrected or missing genes or differentiation factors. For example, expression of GFP-tagged proteins allows cell analysis by flow or another imaging technique. The introduction/knockout/knockdown of proteins can create engineered cells for therapeutical purposes or drug development. In vivo delivery is used in gene therapy in humans, and it can also be used to create or treat animal models.
Disease tends to be dynamic over time, and in the future the spatial and time regulation of target gene expression may become an area of interest. Their increased safety, combined with our current understanding of their advantages and limitations, will allow the use of lentivirus to continue expanding and advancing science.
Conclusions
Lentiviruses have become one of the most attractive and useful tools in research and clinical development. Their applications cover nearly all fields that involve genetic material delivery to cells. Over the years safety concerns have prompted the development of new generations of lentivirus, resulting in SIN and non-integration versions being available. While in essence they are simple tools, the production of high titer pure particles is a multistep, time-consuming and costly process. At BPS Bioscience we offer high purity lentivirus, with titers reflecting the number of actual active viral particles, offer SIN and integrase deficient options, and we can partner with you in custom projects encompassing the complete lentiviral production workflow.
References
(1) Milone M. and O’Doherty U., 2018 Leukemia 32: 1529-1541.
(2) Jmarchn - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=58188472
(3) Dong W. and Kantor B., 2021 Viruses 13(7): 1288.
(4) Martinez-Molina E., et al., 2020 Pharmaceutics 12(11): 1051.
(5) Tucci F., et al., 2020 Molecular Therapy 29 (2): 489-504.

