Therapeutic Targeting of Thymic Stromal Lymphopoietin (TSLP) in Asthma and COPD: A Technical Overview
Introduction
![]() Thymic Stromal Lymphopoietin (TSLP) is a cytokine that plays a crucial role in the pathogenesis of allergic diseases such as asthma and chronic obstructive pulmonary disease (COPD), among others. This technical note provides an overview of the role of TSLP in these respiratory conditions and discusses its implications for therapy. We explore the mechanisms of TSLP in driving airway inflammation and remodeling and review recent advancements in TSLP-targeted therapies for asthma and COPD management.
Thymic Stromal Lymphopoietin (TSLP) is a cytokine that plays a crucial role in the pathogenesis of allergic diseases such as asthma and chronic obstructive pulmonary disease (COPD), among others. This technical note provides an overview of the role of TSLP in these respiratory conditions and discusses its implications for therapy. We explore the mechanisms of TSLP in driving airway inflammation and remodeling and review recent advancements in TSLP-targeted therapies for asthma and COPD management.
Asthma and COPD
Asthma and chronic obstructive pulmonary disease (COPD) are both chronic respiratory conditions characterized by airflow obstruction and respiratory symptoms, but they have distinct underlying mechanisms and clinical presentations.
Asthma is a disease typically characterized by recurrent episodes of wheezing, shortness of breath, chest tightness, and cough, often occurring at night or early morning. It is primarily driven by airway inflammation and hyperreactivity in response to various triggers such as allergens, viral infections, exercise, and pollutants. The inflammation is predominantly eosinophilic, involving the release of cytokines such as interleukin (IL)-4, IL-5, and IL-13, which orchestrate immune cell recruitment, mucus hypersecretion, and airway smooth muscle constriction. Airway remodeling, characterized by structural changes such as thickening of the airway wall, collagen deposition, and increased smooth muscle mass, can occur over time, leading to persistent airflow limitation and worsening of symptoms.
COPD, on the other hand, is primarily caused by exposure to tobacco smoke, although other environmental factors such as biomass fuel exposure and occupational pollutants can also contribute. It is characterized by persistent and progressive airflow limitation that is not fully reversible. COPD encompasses two main phenotypes: chronic bronchitis, characterized by chronic cough and sputum production, and emphysema, characterized by destruction of lung parenchyma and airspace enlargement. The hallmark pathological features of COPD include chronic inflammation, predominantly neutrophilic and macrophage-driven, leading to airway wall thickening, mucus hypersecretion, and loss of lung elastic recoil. Additionally, COPD is associated with systemic inflammation and comorbidities such as cardiovascular disease, skeletal muscle dysfunction, and osteoporosis, which contribute to its significant morbidity and mortality.
Despite the availability of treatments, many patients continue to experience uncontrolled symptoms, highlighting the need for novel therapeutic approaches. TSLP has emerged as a key regulator of airway inflammation and remodeling in both asthma and COPD, offering a promising targeted approach for therapeutic intervention.
The Biology of TSLP
TSLP is a type I cytokine that is a paralogue to IL-7, likely derived from the same ancestral gene (1). It is produced mainly by epithelial cells and stromal cells in the lungs, skin, and gastrointestinal tract. A variety of triggers can induce TSLP production, including injury, ligands for TLR2 (Toll-Like Receptor 2), TLR3, and NOD2, helminth, bacterial and viral infections, proteases such as trypsin and papain, and pro-inflammatory cytokines (2). TSLP effectively acts as an alarmin molecule that amplifies inflammatory defense mechanisms.
TSLP binds to the heterodimeric receptor composed of TSLPR (thymic stromal lymphopoietin receptor) and IL-7 receptor alpha-chain (IL-7Rα), initiating a cascade of phosphorylation mediated by JAK1 and JAK2 (3). Subsequent recruitment and activation of STAT5A and STAT5B ultimately lead to the transcription and production of the Th2-related cytokines IL-4, IL-5, IL-9, and IL-13. TSLPR is expressed by a variety of immune cells, including T cells, B cells, innate lymphoid cells (ILC2s), Natural killer T (NKT) cells, monocyte/macrophages, basophils, dendritic cells (DCs), as well as non-immune cells such as epithelial cells, smooth muscle cells and nerve cells.
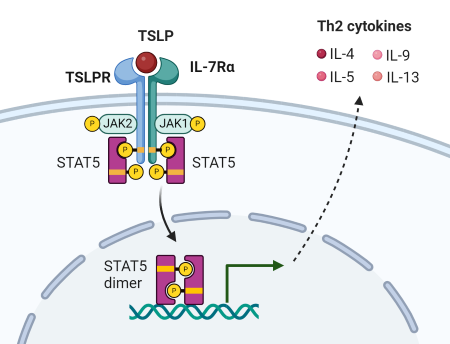
Figure 1. TSLP signaling through heterodimeric receptor: TSLPR and IL-7Rα. Adapted from Ebina-Shibuya, R. and Leonard, WJ. 2022 (1).
The Role of TSLP in Asthma and COPD
TSLP is produced by epithelial cells in response to various triggers such as allergens, viruses, and pollutants. It acts as a master regulator of type 2 inflammation by promoting the activation and differentiation of dendritic cells, which in turn drive the differentiation of naïve T cells into pro-inflammatory Th2 cells. Th2 cytokines, including IL-4, IL-5, and IL-13, orchestrate eosinophilic inflammation, mucus hypersecretion, and airway hyperresponsiveness, hallmark features of asthma. Additionally, TSLP contributes to airway remodeling by promoting the production of extracellular matrix proteins and fibroblast activation. Thus, targeting TSLP signaling pathways holds promise for mitigating both inflammation and remodeling in asthma.
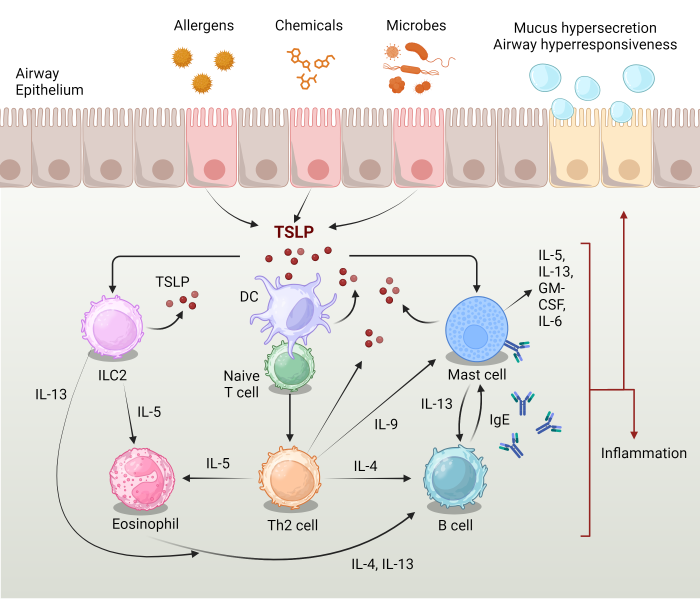
Figure 2. TSLP drives Th2 inflammatory pathways in asthma. Adapted from Ebina-Shibuya, R. and Leonard, WJ. 2022 (1).
In COPD, TSLP expression is upregulated in response to cigarette smoke exposure and respiratory infections. TSLP contributes to chronic inflammation by recruiting and activating innate immune cells, including neutrophils and macrophages, leading to the production of pro-inflammatory mediators. Moreover, TSLP induces airway smooth muscle proliferation and collagen deposition, contributing to airflow limitation and lung function decline in COPD. Targeting TSLP pathways presents a novel approach for addressing inflammation and remodeling in COPD and potentially slowing disease progression.
Therapeutic Implications
Several therapeutic strategies targeting TSLP are being explored for the treatment of asthma and COPD. Monoclonal antibodies against TSLP or its receptor have shown efficacy in preclinical studies and early-phase clinical trials, demonstrating improvements in lung function, symptoms, and exacerbation rates. Small molecule inhibitors of TSLP signaling pathways are also under development and hold promise as oral therapies for long-term disease management. Combination therapies targeting multiple inflammatory pathways, including TSLP, may offer synergistic effects and improve treatment outcomes in severe asthma and COPD phenotypes. Targeting TSLP signaling pathways represents a promising therapeutic approach for these respiratory diseases, offering the potential to alleviate symptoms, improve lung function, and modify disease progression. Further research is warranted to fully elucidate the complex mechanisms of TSLP and optimize therapeutic strategies for clinical implementation.
BPS Bioscience Solutions Enabling TSLP Drug Discovery
TSLP Responsive Luciferase Reporter Ba/F3 Cell Line
This cell line is a murine Ba/F3 cell line engineered to express both TSLPR and IL-7Rα (Interleukin 7 receptor alpha) separated by a self-cleaving P2A peptide. The construct was delivered by transduction of STAT5 Luciferase Reporter Ba/F3 cells (#79772), which express a firefly luciferase reporter driven by STAT5 response elements located upstream of the minimal TATA promoter. After activation by TSLP, the endogenous transcription factor STAT5 binds to the response elements, inducing transcription of the luciferase reporter gene. The cell line has been validated to respond to TSLP. It does not respond to IL-7. Additional functional validation demonstrates that TSLP-induced luciferase activity can be inhibited by either anti-TSLP or anti-TSLPR neutralizing antibodies.
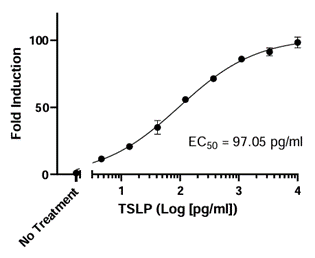
Figure 3. Dose-response to TSLP in the TSLP Responsive Luciferase Reporter Ba/F3 Cell Line.
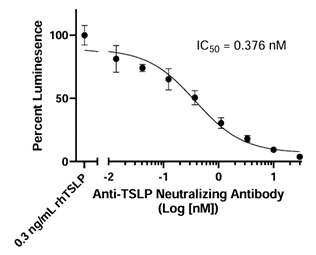
Figure 4. Inhibition of TSLP response by anti-TSLP Neutralizing Antibody in the TSLP Responsive Luciferase Reporter Ba/F3 Cell Line. TSLP Responsive Luciferase Reporter Ba/F3 cells were incubated with increasing concentrations of Anti-TSLP Neutralizing Antibody (#102138) overnight before stimulation with 0.3 ng/ml of TSLP. Luciferase activity was measured using ONE-Step™ Luciferase Assay System (#60690). Results are shown as percentage of STAT5 reporter activity (compared to cells stimulated by TSLP in the absence of antibody, set at 100%).
To quickly screen and validate blockers of TSLP:TSLPR interaction, the TSLPR:TSLP [Biotinylated] Inhibitor Screening Chemiluminescence Assay Kit is an ideal choice. This ELISA-based chemiluminescent assay provides robust signal-to-noise ratios and is provided in a convenient kit format. Quality testing ensures lot-to-lot consistency in performance.
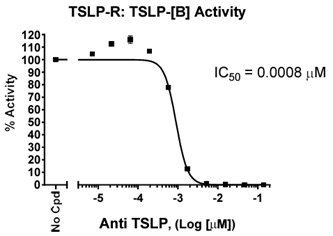
Figure 5. Dose-dependent inhibition of TSLP:TSLPR binding by Anti-TSLP Neutralizing Antibody (#102138).
References
1. Ebina-Shibuya, R. and Leonard, WJ. 2022. Nat Rev Immunol. 23:24-37. Pubmed
2. Takai, T. 2012. Allergol Int. 61:3-17. Pubmed
3. Ziegler, S. 2012. J Allergy Clin Immunol. 130:845-852. Pubmed
4. Figures 1 and 2 were created with BioRender.com.
│Related Products
- TSLP Responsive Luciferase Reporter Ba/F3 Cell Line
- TSLPR:TSLP [Biotinylated] Inhibitor Screening Chemiluminescence Assay Kit
- Anti-TSLP Neutralizing Antibody
- TSLP and TSLPR Recombinant Proteins

