TL1A:DR3 - A Pathway for Immunomodulation
Introduction
![]() The TL1A:DR3 pathway stands as a promising frontier in immunomodulation, offering insights into novel therapeutic interventions for various autoimmune diseases, inflammatory disorders, and cancer. This technical note explores the intricate mechanisms underlying TL1A:DR3 signaling, its physiological roles, and its potential applications in clinical settings. By elucidating the complexities of this pathway, researchers and clinicians can harness its therapeutic potential to revolutionize the treatment landscape for immune-related ailments.
The TL1A:DR3 pathway stands as a promising frontier in immunomodulation, offering insights into novel therapeutic interventions for various autoimmune diseases, inflammatory disorders, and cancer. This technical note explores the intricate mechanisms underlying TL1A:DR3 signaling, its physiological roles, and its potential applications in clinical settings. By elucidating the complexities of this pathway, researchers and clinicians can harness its therapeutic potential to revolutionize the treatment landscape for immune-related ailments.
The Biology of TL1A and DR3
TL1A, also known as vascular endothelial growth inhibitor (VEGI)-251 or TNFSF15, is a member of the tumor necrosis factor (TNF) superfamily. TL1A and its receptor, death receptor 3 (DR3), also known as APO-3, LARD, TRAMP, and WSL-1, have garnered attention for their pivotal roles in regulating immune responses. The TL1A:DR3 pathway serves as a crucial mediator in the crosstalk between innate and adaptive immunity, influencing immune cell activation, differentiation, and cytokine production.
TL1A is expressed as a trimeric type II transmembrane protein that is subsequently released as a soluble protein through the actions of metalloproteinase enzymes such as TNF-α converting enzyme (TACE). It binds to its receptor, DR3, initiating downstream signaling cascades that culminate in diverse cellular responses. Multiple pathways lead to inflammation, apoptosis, or necrosis. TL1A binding activates DR3 signaling via TNFR-associated death domain protein (TRADD), triggering the recruitment of adaptor proteins and kinases, such as TRAF2 and RIP1, which leads to the activation of transcription factors such as NF-κB and AP-1. Subsequent gene expression alterations drive the proliferation of T cells, the production of pro-inflammatory cytokines, and the differentiation of T helper cell subsets. Additionally, TL1A:DR3 signaling influences the function of regulatory T cells (Tregs), further shaping immune homeostasis. Alternatively, DR3 can signal via Fas-associated death domain (FADD), recruiting cytoplasmic molecules RIP1 and RIP3, which activate caspase pathways, inducing apoptosis or necroptosis.
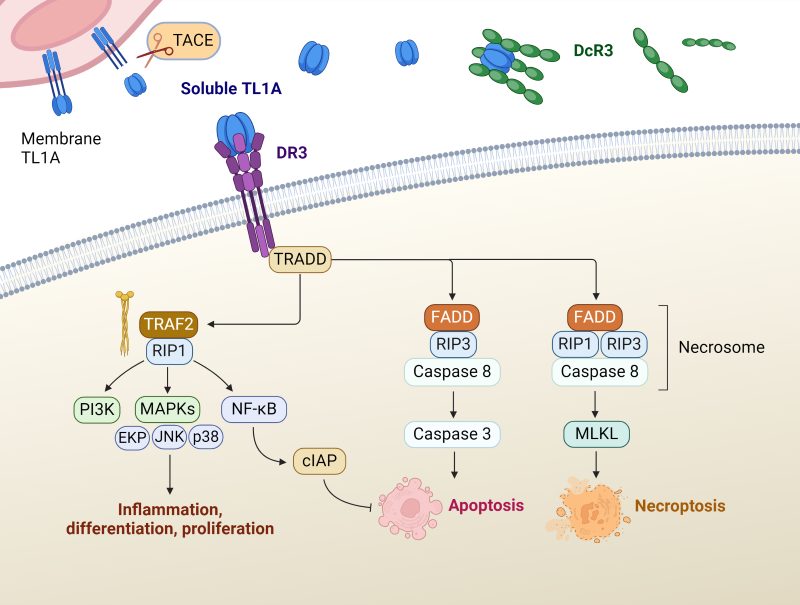
Figure 1. Signaling pathways of TL1A:DR3. Adapted from Xu, WD, et.al. 2022 (1).
Decoy receptor 3 (DcR3), a soluble member of the TNF receptor superfamily, intricately modulates TL1A biology by acting as a competitive inhibitor, binding to TL1A and preventing its interaction with the functional receptor, DR3. Through this mechanism, DcR3 effectively dampens TL1A-mediated signaling pathways, thereby attenuating immune responses, and contributing to immune regulation and tolerance. Additionally, DcR3 has been implicated in the pathogenesis of autoimmune diseases and cancer, where its dysregulation may disrupt immune homeostasis and promote disease progression.
The TL1A:DR3 pathway exerts pleiotropic effects across various physiological contexts, including immune tolerance, tissue repair, and host defense. In health, it contributes to immune surveillance and the resolution of infections by orchestrating appropriate immune responses. However, dysregulation of TL1A:DR3 signaling is implicated in the pathogenesis of autoimmune diseases such as rheumatoid arthritis, inflammatory bowel disease, and psoriasis, highlighting its significance in immune-mediated disorders.
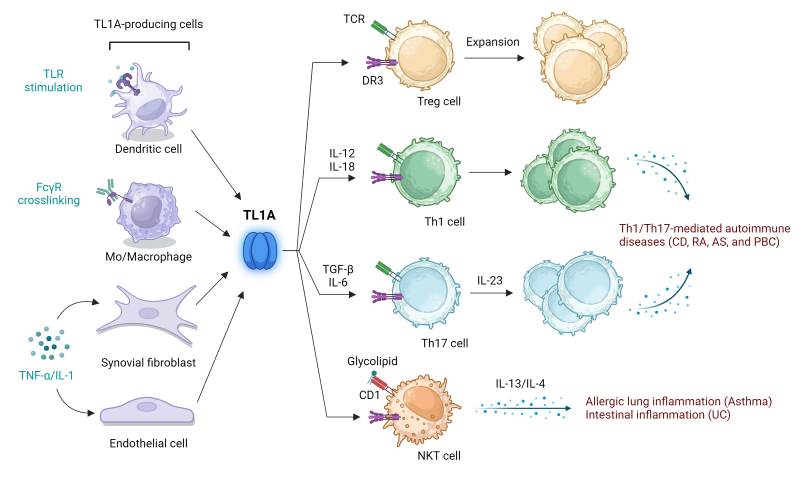
Figure 2. TL1A bridges innate and adaptive immune responses and is a key driver of autoimmune and inflammatory pathologies, including Crohn’s Disease (CD), rheumatoid arthritis (RA), ankylosing spondylitis (AS), primary biliary cirrhosis (PBC), asthma, and ulcerative colitis (UC). Adapted from Aiba, Y and Nakmura, M, 2013 (2).
Therapeutic Implications
Targeting the TL1A:DR3 pathway holds immense therapeutic promise for mitigating aberrant immune responses in autoimmune and inflammatory conditions. Pharmacological interventions aimed at modulating TL1A activity or blocking DR3 signaling, primarily using antibodies, have shown encouraging results in preclinical studies and early-phase clinical trials. By selectively manipulating this pathway, it may be possible to restore immune balance and ameliorate disease severity with improved specificity and reduced adverse effects compared to conventional immunosuppressive therapies.
On the other hand, co-stimulation of exhausted cells using DR3 agonists may be beneficial in tumor environments where T cell function is diminished (3). While it is evident that blocking immune checkpoint inhibitor interactions, such as PD-1: PD-L1, can restore T cell function, other strategies may be needed to address cases where immune checkpoint blockade therapy fails. Furthermore, a combination of immune checkpoint blockade with DR3 agonists may provide enhanced effectiveness over single therapies alone.
Innovations for Drug Discovery
BPS Bioscience enables drug discovery by offering critical solutions for research on the TL1A:DR3 pathway.
TL1A-Responsive Luciferase Reporter Jurkat Cell Line
Anti-TL1A Neutralizing Antibody
TL1A, His-Tag, Avi-Tag Recombinant Protein
The cell line is a TL1A-responsive DR3/NF-κB luciferase reporter Jurkat cell line expressing firefly luciferase under the control of an NF-κB response element with stable expression of human DR3. Expression of the firefly luciferase reporter is driven by NF-κB response elements located upstream of the minimal TATA promoter. Activation of the NF-κB signaling pathway by the DR3 ligand TL1A can be monitored by measuring luciferase activity. This stable cell line is ideal for testing and validating blockers of TL1A or DR3, or for investigating DR3 cell signaling. This cell line has been validated to be responsive to TL1A and TNF-α stimulation. Recombinant TL1A (#101880) stimulation was blocked by anti-TL1A neutralizing antibody (#101729) and DcR3 (decoy receptor 3).
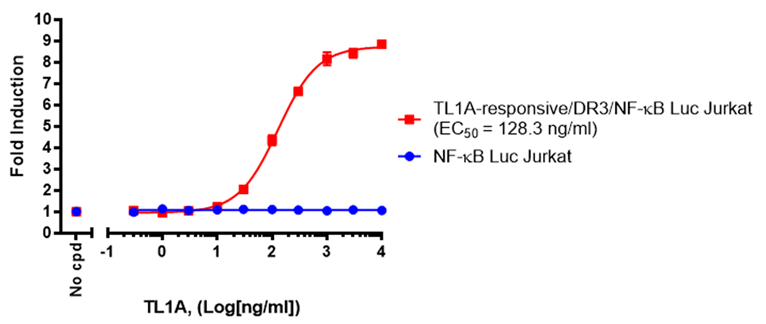
Figure 3. Dose-response to TL1A in the TL1A Responsive Luciferase Reporter Jurkat Cell Line compared to a negative control cell line that does not express DR3.
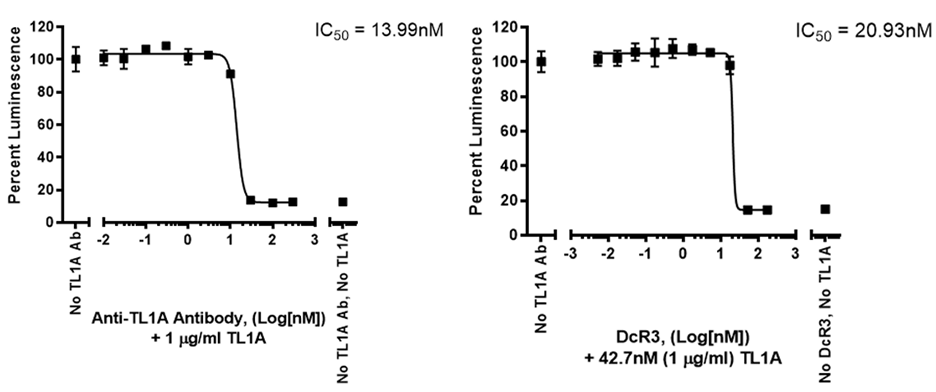
Figure 4. Inhibition of TL1A response by anti-TL1A Antibody and DcR3 Fc chimera in the TL1A Responsive Luciferase Reporter Jurkat Cell Line.
Biochemical assay kits enable screening and validation of inhibitors for DR3:TL1A and DcR3:TL1A interactions. These ELISA-based chemiluminescent assays provide robust signal-to-noise ratios and are provided in a convenient kit format. Quality testing ensures lot-to-lot consistency in performance.
DR3:TL1A Inhibitor Screening Assay Kit
DcR3:TL1A Inhibitor Screening Assay Kit
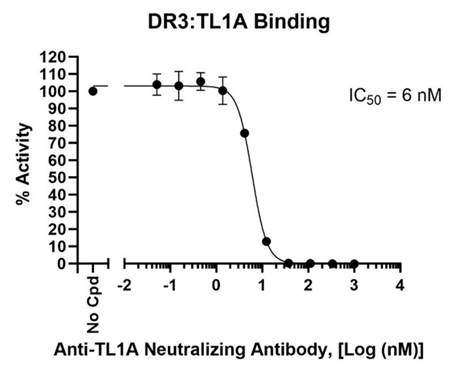
Figure 5. Dose-dependent inhibition of DR3:TL1A binding by Anti-TL1A Neutralizing Antibody (#101729).
References
1. Xu, WD., et.al. 2022. Front Immunol. 13:891328. Pubmed
2. Aiba, Y., and Nakamura, M. 2013. Mediators Inflamm. 2013:258164. Pubmed
3. Zwolak, A. et.al. 2022. Sci Rep. 12(1):20538. Pubmed
4. Figures 1 and 2 were created with BioRender.com.
│Related Products
- TL1A-Responsive Luciferase Reporter Jurkat Cell Line
- Anti-TL1A Neutralizing Antibody
- TL1A, His-Tag, Avi-Tag Recombinant Protein
- DR3:TL1A Inhibitor Screening Assay Kit
- DcR3:TL1A Inhibitor Screening Assay Kit

