SARS-CoV-2 Antibody Detection
SARS-CoV-2 has spread across the globe effectively at a rapid pace causing a worldwide pandemic. Infection with this highly contagious respiratory virus can be asymptomatic or present as COVID-19, a disease with varying degrees of severity. Clinical symptoms may include fever, dry cough, anosmia, gastrointestinal symptoms, hypercoagulability, hyperimmune response (cytokine storm), and acute respiratory distress syndrome (ARDS) with disease progression (1). Patients with underlying health conditions such as CVD, hypertension, diabetes, and COPD are likely to have worse clinical outcomes, including multi-organ failure and death.
Due to the rapidly evolving nature of this pandemic, the true extent of spread of SARS-CoV-2 will likely not fully be realized until after the pandemic. Moreover, as observed in all respiratory viral pandemics since the 1918 Influenza pandemic, the true number of infections always exceeds the detected cases (2). Since emergence of SARS-CoV-2, there has been a critical need to understand prevalence, transmission patterns, calculate the burden of disease and case fatality rates (3). Molecular diagnostic testing methods, which are suitable for viral genome detection, are not ideal for determining true case counts and rates of asymptomatic infection. Therefore, serological detection of SARS-CoV-2 specific antibodies can contribute to filling these knowledge gaps. Serological testing is also vital for the assessment of SARS-CoV-2 exposure, infection, and potential immunity from COVID-19.
BPS Bioscience IgG Detection Assay Kits
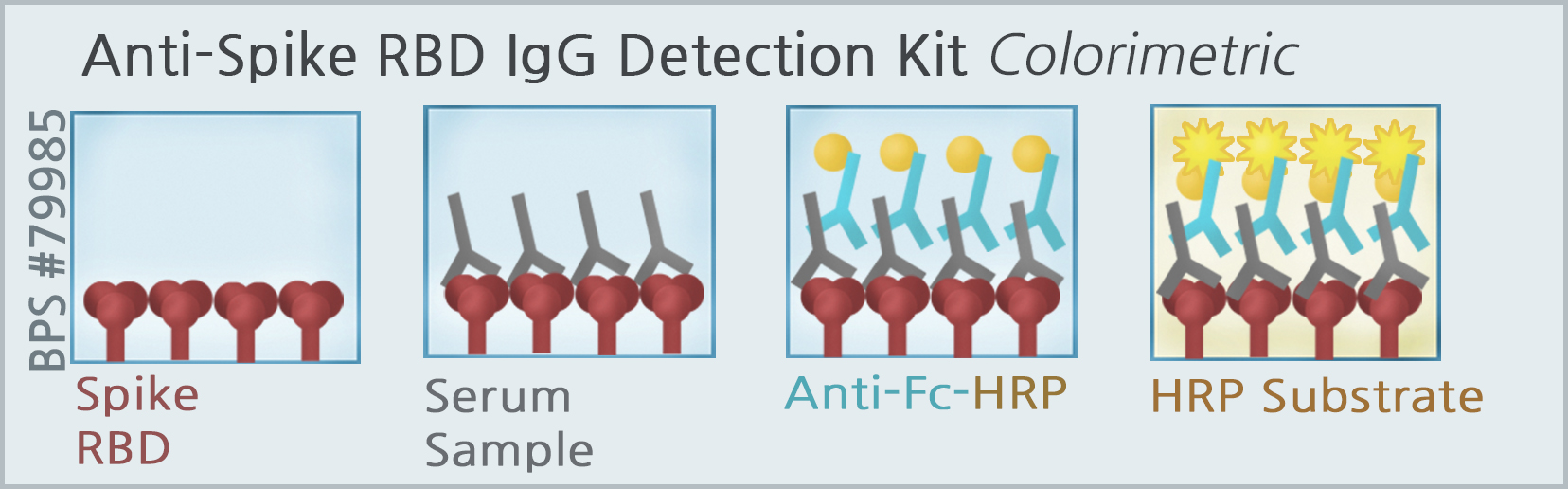
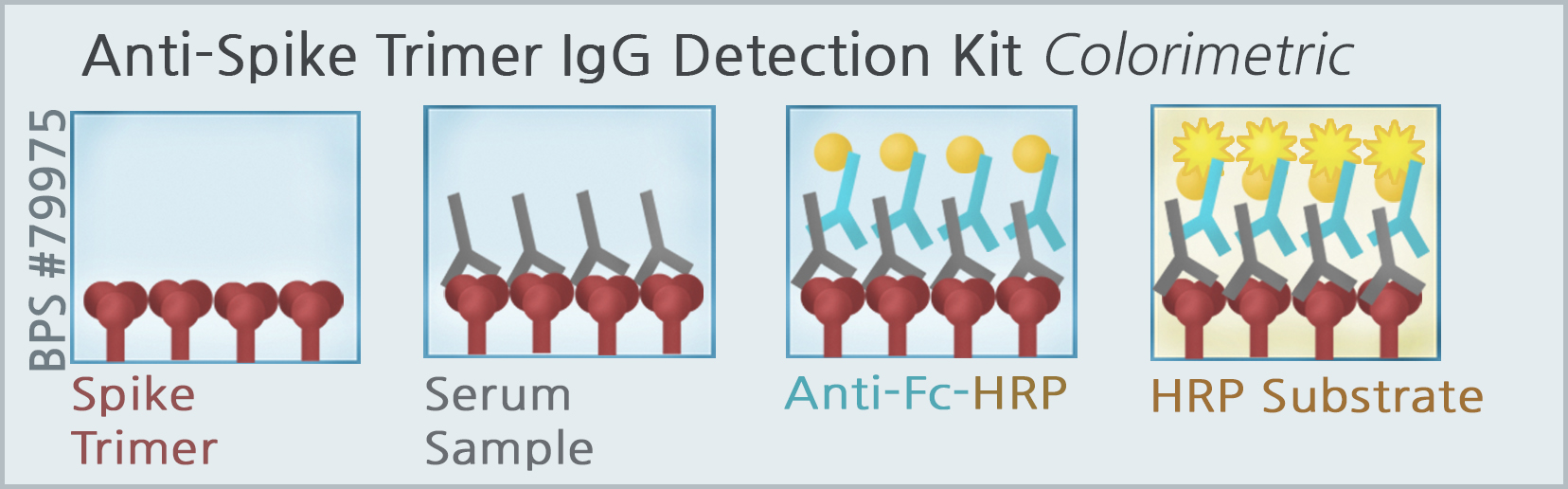
BPS Bioscience has developed and optimized a SARS-CoV-2-specific-enzyme linked immunosorbent assay (ELISA) in colorimetric (BPS #79975 & #79985) and chemiluminescence formats for qualitative detection of IgG by using the full-length SARS-CoV-2 spike trimeric protein (BPS #100728), and the Receptor Binding Domain (RBD) of the S1 polypeptide subunit (BPS #100696). The 96-well assay plate is coated with either the spike trimeric protein or RBD of S1 spike, and incubated with normal human serum spiked with a concentration gradient of human IgG that is specific to the spike protein coated in the assay plate. The antigen-antibody complex is detected by enzyme tagged anti-human Fc-HRP conjugate secondary antibody and HRP colorimetric or chemiluminescence substrate. The intensity of the color change measured at A450, or the gain in luminescence signal is proportional to the amount of antigen-antibody complexes formed in the assay. These assay kits are designed for research use only.
Antibodies and Viral Infection Prevention
Antibodies, also known as immunoglobulins are glycosylated protein molecules present on the surface of B cells (surface immunoglobulins). They serve as antigen receptors or are secreted into the extracellular space where they can bind and neutralize their target antigens (4). An antibody molecule consists of 4 protein chains: 2 “heavy” (H chains) and 2 “light” (L chains) linked to each other by disulfide bonds. The N terminal regions of the heavy and light chains, which collectively make up the antigen-binding site determines antibody specificity. There are 5 isotypes or classes of antibodies (IgM, IgD, IgG, IgA, and IgE), and they are distinguished according to the C-terminal regions (constant regions) of the heavy chains. The constant region, does not participate in antigen binding. Instead, they are important for the common effector functions of antibodies.
Antibodies are found in plasma and in extracellular fluid. Antibodies have 3 primary functions (5).
- Antibodies are secreted into the blood and mucosa, where they bind to and inactivate foreign substances such as pathogens and toxins. This process is known as antibody neutralization, and it is important for the prevention of viruses binding and infecting host cells. Antibodies can neutralize viral infectivity in a number of ways, as summarized in the figure below.
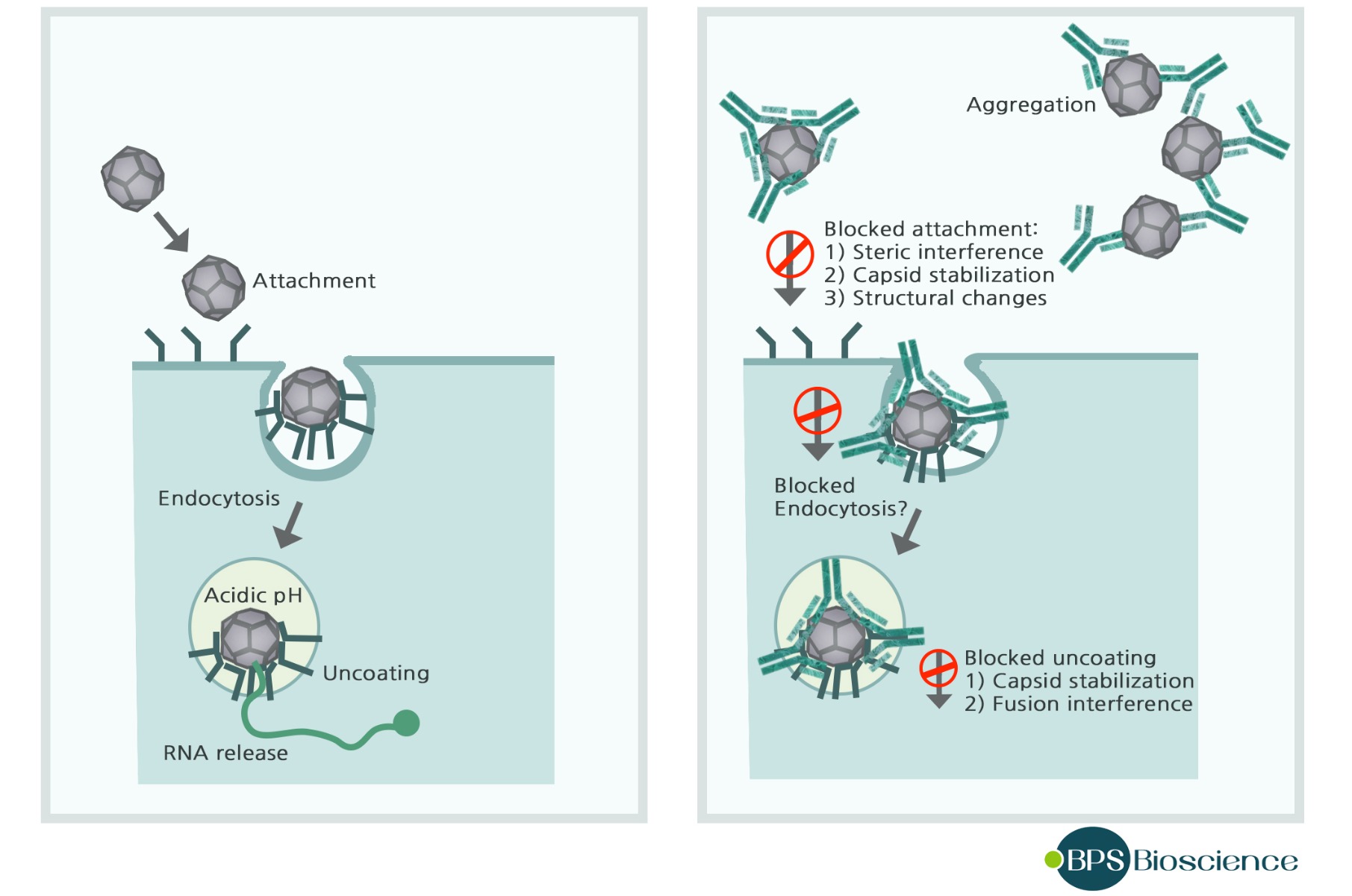
Antibodies may interfere with virus binding to receptors, block uptake into cells, prevent uncoating of the genomes in endosomes, or cause aggregation of virus particles. Many enveloped viruses are lysed when antiviral antibodies and serum complement disrupt membranes (6). - Non-neutralizing antibodies are also produced after viral infection. Such antibodies facilitate phagocytosis of foreign substances by phagocytic cells such as monocytes, macrophages, granulocytes, and dendritic cells. This process is known as opsonization, which involves antibody-mediated phagocytosis.
- The third function is antibody activation of the complement system to destroy pathogens through proteolysis and enhanced chemotaxis.
SARS-CoV-2 infection and detection of IgG and IgM Antibodies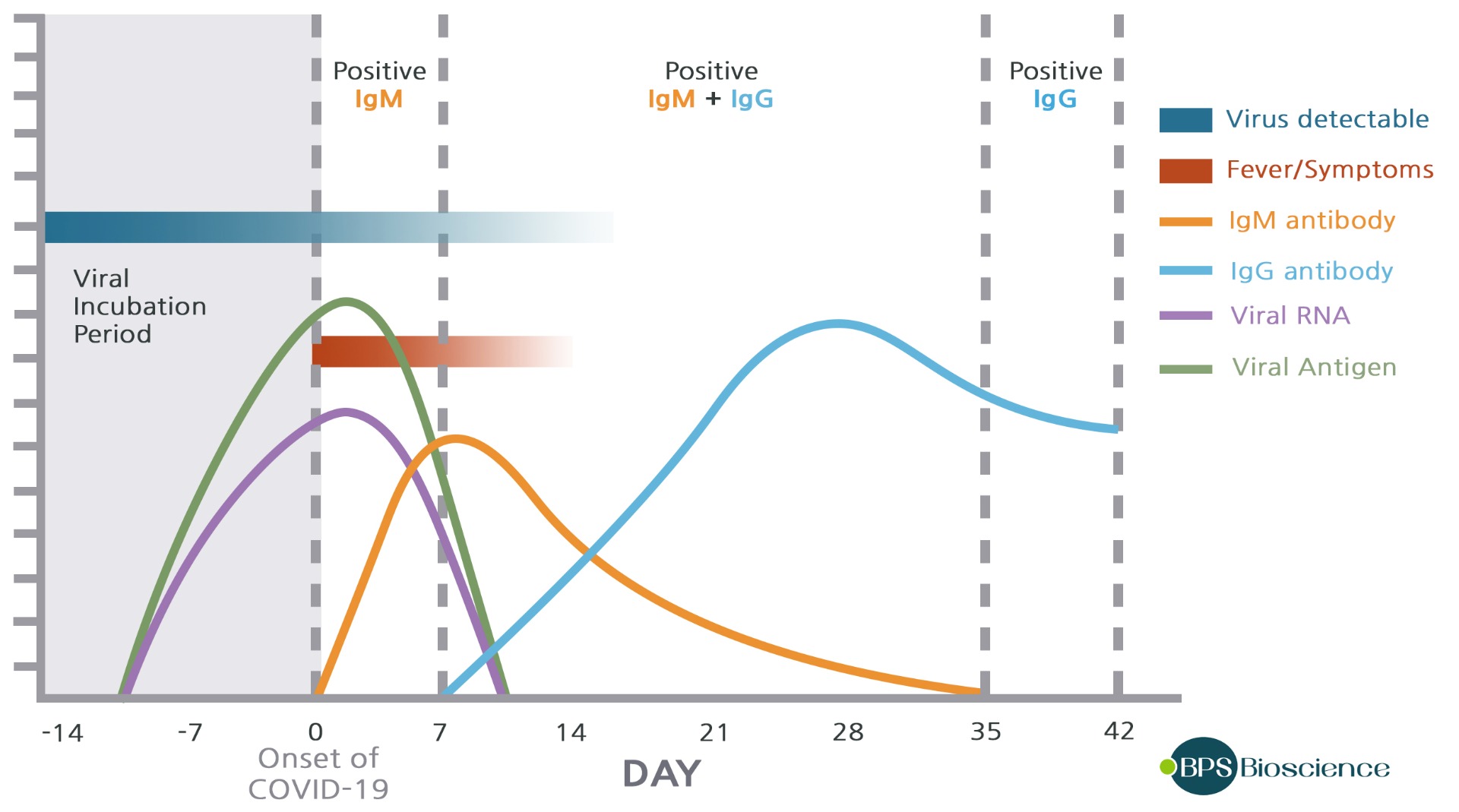
After initial exposure to SARS-CoV-2, the IgM levels in blood plasma increase during the first week and then peak by the second week before reducing to near‐background levels in most patients. Whereas IgG, which is normally detectable after 1 week, is maintained at high levels for a long time period. The detection of these antibodies is essential to determine seroconversion, immune status, and recovery in individuals exposed to the virus.
Relevant Products
- Anti-Human IgG, Unconjugated Antibody (Negative Control)
- Anti-Human IgM, Unconjugated Antibody
- Anti-Spike S1 Monoclonal Antibody (SARS-CoV-2)
References
- Guan, W. J. et al. (2020) N. Engl. J. Med. doi:10.1056/NEJMoa2002032.
- Taubenberger, J. K. et al. (2006) Emerg. Infect. Dis. doi:10.3201/eid1201.050979.
- Freeman et al. BioRxiv-CHL (2020) doi.org/10.1101/2020.04.24.057323
- Murphy K.P. 8th. Taylor & Francis Group; New York, NY: 2012. Janeway’s Immunobiology.
- Jackofsky, D.,et al. J Arthroplasty. 2020 Apr 27 doi: 10.1016/j.arth.2020.04.055
- Racaniello, V. 24 July 2009 Virology Blog www.virology.ws/2009/07/24/virus-neutralization-by-antibodies/