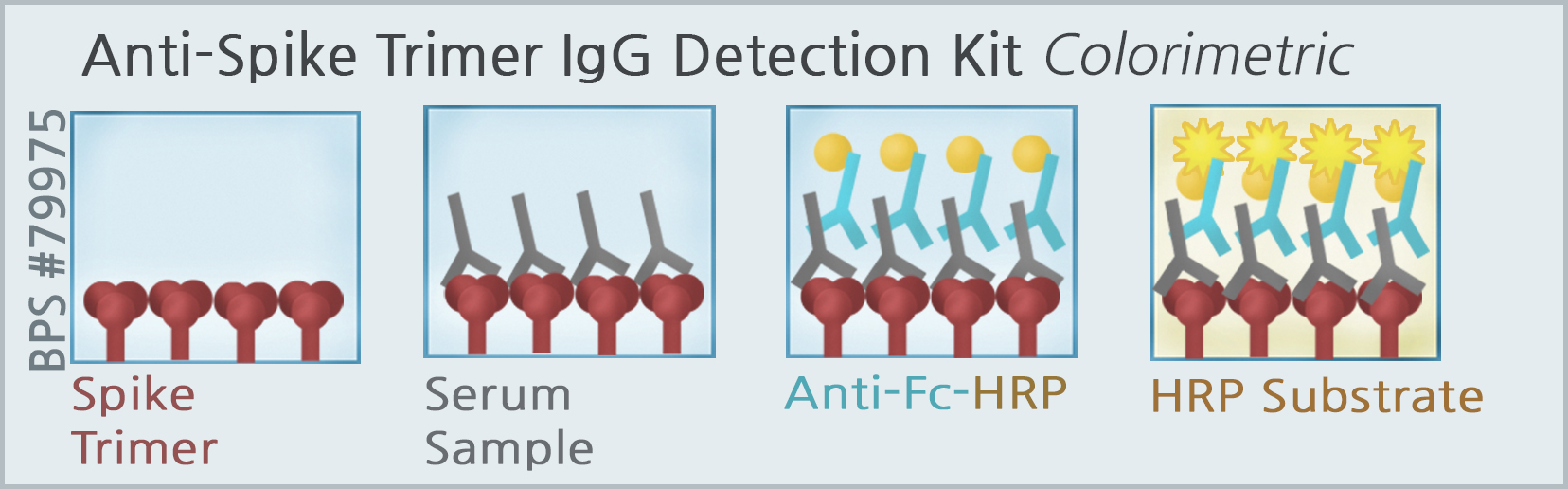
SARS-CoV-2 IgG Detection Kit (Colorimetric Trimer Anti-Spike IgG detection)
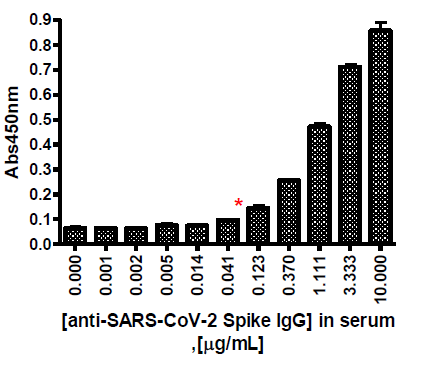
The SARS-CoV-2 IgG detection kit is designed for qualitative detection of human IgG antibodies in serum collected from individuals suspected of prior infection with the virus that causes COVID-19. This fast and simple ELISA uses the trimeric form of the SARS-CoV-2 Spike protein (BPS Bioscience #100728) to identify IgG antibodies that indicate a previous infection with SARS-CoV-2. The Spike protein is expressed on the viral membrane as a trimer, which means this kit measures IgG antibodies in a more physiologically relevant context than many other commercially available ELISA kits.
PBS (Phosphate buffered saline)
PBST (Phosphate buffered saline containing 0.05% Tween-20)
Dry Milk (Fisher #115668 or compatible)
1N HCl (aqueous)
Rotating or rocker platform
UV/Vis spectrophotometer microplate reader capable of reading absorbance at 450 nm*
| Catalog Number |
Component |
Amount |
Storage |
|
| 100728 | Spike Trimer (S1 + S2), His-Tag (SARS-CoV-2) | 5 µg | -80°C | Avoid mulitple freeze/ thaaw cycles! |
| anti-human Fc-HRP conjugate (1 mg/ml) | 5 µl | +4°C | ||
| Colorimetric HRP substrate | 10 ml | +4°C | ||
| 79964 | Transparent 96-well microplate | 1 | Room Temp |
|
*The initial concentration of Spike Trimer is lot-specific and will be indicated on the tube containing the protein.
As the viral load increases in the infected individual prior to the onset of symptoms, the individual may unknowingly be able to actively spread the infection during this presymptomatic phase. Once the immune system recognizes the infection, IgM is generated against the virus initially, followed by a second response leading to the production of higher affinity IgG molecules targeting the SARS-CoV-2 virus. IgG antibodies to SARS-CoV-2 generally become detectable beginning 10 – 14 days following infection but may occur later. The presence of IgG antibodies, following previously negative testing defines IgG antibody seroconversion following SARS-CoV-2 infection. False positive results for IgG antibodies may occur due to cross-reactivity from pre-existing antibodies or other possible causes. This assay kit is not intended for clinical diagnostic use.
Long, Q.-X., et al. 2020. Antibody responses to SARS-CoV-2 in patients withCOVID-19. Nat. Med. (in press). https://doi.org/10.1038/s41591-020-0897-1