Spike (SARS-CoV-2) Pseudotyped Lentivirus (Luciferase Reporter)
The pandemic coronavirus disease 2019 (COVID-19) is caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). As the first step of the viral replication, the virus attaches to the host cell surface before entering the cell. The viral Spike protein recognizes and attaches to the Angiotensin-Converting Enzyme 2 (ACE2) receptor found on the surface of type I and II pneumocytes, endothelial cells, and ciliated bronchial epithelial cells. Drugs targeting the interaction between the Spike protein of SARS-CoV-2 and ACE2 may offer protection against the viral infection.
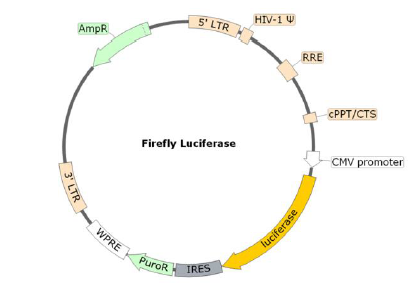
The SARS-CoV-2 Spike Pseudotyped Lentivirus were produced with SARS-CoV-2 Spike (Genbank Accession #QHD43416.1) as the envelope glycoproteins instead of the commonly used VSV-G. These pseudovirions also contain the firefly luciferase gene driven by a CMV promoter (Figure 1), therefore, the spike-mediated cell entry can be conveniently measured via luciferase reporter activity. The SARS-CoV-2 Spike pseudotyped lentivirus can be used to measure the activity of neutralizing antibody against SARS-CoV-2 in a Biosafety Level 2 facility.
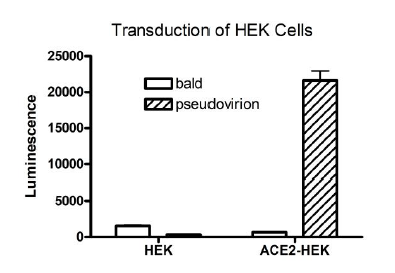
Spike interacts with host cells by binding to membrane receptor ACE2 (angiotensin converting enzyme 2). Based on experiments performed by scientists at BPS Bioscience, we know that SARS-CoV-2 spike pseudotyped lentiviruses transduce the following cells with great efficiency: ACE2-HEK293 cells (BPS Bioscience, #79951), ACE2-CHO cells (BPS Bioscience, #79959), ACE2-HeLa cells (BPS Bioscience, #79958). They also efficiently transduce TMPRSS2-Vero E6 cells (BPS Bioscience, #78081), which express high endogenous levels of ACE2 and were stably transfected with human serine protease TMPRSS2 required for the priming of Spike and fusion of the virion with the plasma membrane. By contrast, the SARS-CoV-2 spike pseudotyped lentivirus does not transduce parental Calu3 and Vero E6 cells with great efficiency, as previously demonstrated by others [Neerukonda et al. 2021, PlosOne PMID: 33690649; Tandon et al. 2020, Scientific Reports PMID: 33154514; Condor Capcha et al. 2021, Front. Cardiovasc. Med. PMID: 33521067; Pisil et al. 2021, Pathogens PMID: 33540924].
As recommended in our protocol, 5 µl of virus/well in a 96-well plate provides a sufficient signal-to-noise ratio to perform inhibition studies. The amount of virus added to the cells can also be scaled down according to the user’s need.
Figure 1. Schematic of the Luciferase Reporter in SARS-CoV-2 Spike Pseudovirion
This product has been cited 15 times.
| Name | Ordering Information |
| Thaw Medium 1 or HEK293 Growth Medium | BPS Bioscience, #60187 |
| ACE2-HEK293 Recombinant Cell Line | BPS Bioscience, #79951 |
| Bald Lentiviral Pseudovirion (Luciferase Reporter) | BPS Bioscience, #79943 |
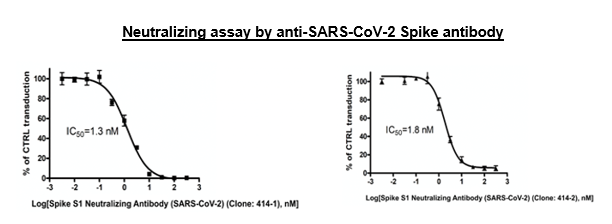
| Spike S1 Neutralizing Antibody (SARS-CoV-2) (Clone: 414-1) | BPS Bioscience, #100793 |
| Spike S1 Neutralizing Antibody (SARS-CoV-2) (Clone: 414-2) | BPS Bioscience, #100792 |
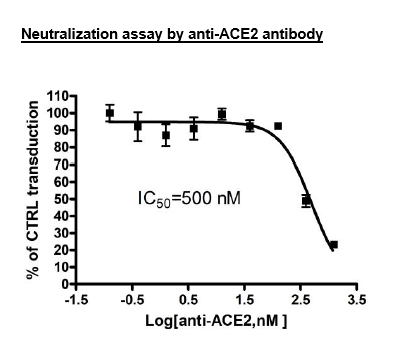
| Anti-ACE2 Antibody | R&D Systems, #AF933 |
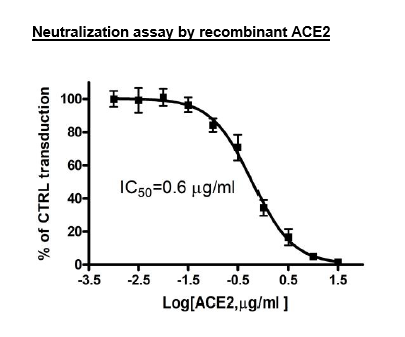
| Recombinant ACE2 protein | BPS Bioscience, #11003 |
| 96-well tissue culture treated, white clear-bottom assay plate | Corning, #3610 |
| ONE-Step™ Luciferase Assay System | BPS Bioscience, #60690 |
The lentiviruses were produced from HEK293T cells in medium containing 90% DMEM + 10% FBS.