Spike (BA.4/5, Omicron Variant) (SARS-CoV-2) Pseudotyped VSV Delta G (Luciferase Reporter)
The Spike protein of BA.4 and BA.5 variants of SARS-CoV-2 have identical mutations. In this datasheet, the spike protein of BA.4 and BA.5 are referred to as BA.4/5.
The Spike (BA.4/5, Omicron Variant) (SARS-CoV-2) Pseudotyped VSV Delta G (Luciferase Reporter) was produced with SARS-CoV-2 Spike (Genbank Accession #QHD43416.1 containing all the Omicron BA.4/5 mutations; see below for details) as the envelope glycoprotein instead of VSV-G. The pseudovirions contain the firefly luciferase gene, therefore, the spike-mediated cell entry can be measured via luciferase activity. The Spike (BA.4/5 Variant) (SARS-CoV-2) Pseudotyped VSV Delta G (Luciferase Reporter) can be used to measure the activity of a neutralizing antibody against SARS-CoV-2 BA.4/5 variant in a Biosafety Level 2 facility.
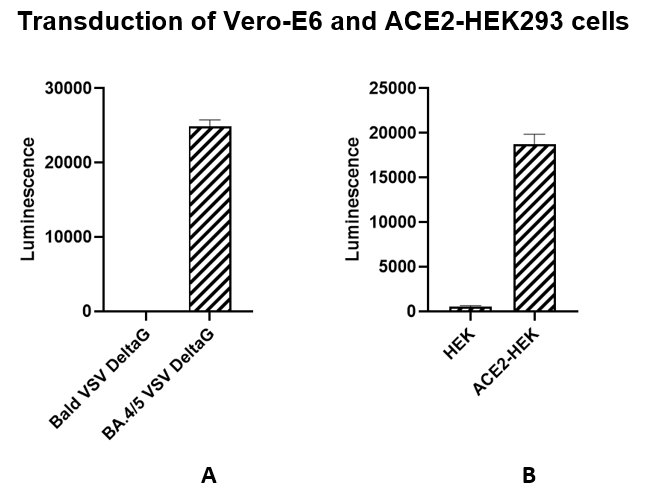
As shown in Figures 1 and 2, the Spike (BA.4/5 Variant) (SARS-CoV-2) Pseudotyped VSV Delta G (Luciferase Reporter) has been validated for use with target cells Vero-E6 and ACE2-HEK293 (BPS Bioscience #79951). Spike VSV Delta G is preferred for use in cells such as Vero-E6 and Calu-3.
Spike Mutations in BA.4/5, Omicron Variant:
Del69-70, T19I, LPPA24-27S, G142D, V213G, G339D, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, L452R, S477N, T478K, E484A, F486V, Q498R, N501Y, Y505H, D614G, H655Y, N679K, P681H, N764K, D796Y, Q954H, N969K
| Name | Ordering Information |
| Thaw Medium 1 | BPS Bioscience #60187 |
| Bald VSV Delta G (Luciferase reporter) | BPS Bioscience #78636 |
| Vero-E6 | ATCC #CRL-1586 |
| ACE2-HEK293 Recombinant Cell Line | BPS Bioscience #79951 |
| Spike Neutralizing Antibody (Clone G10xA1) (SARS-CoV-2) | BPS Bioscience #101326 |
| ONE-Step™ Luciferase Assay System | BPS Bioscience #60690 |
| 96-well tissue culture treated, white clear-bottom assay plate | Corning, #3610 |
The pseudoviruses were produced from HEK293T cells. They are supplied in cell culture medium containing 90% DMEM + 10% FBS.
The pandemic coronavirus disease 2019 (COVID-19) is caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). As the first step of the viral replication, the virus attaches to the host cell surface before entering the cell. The viral Spike protein recognizes and attaches to the Angiotensin-Converting Enzyme 2 (ACE2) receptor found on the surface of type I and II pneumocytes, endothelial cells, and ciliated bronchial epithelial cells. Drugs targeting the interaction between the Spike protein of SARS-CoV-2 and human ACE2 may offer protection against viral infection. The Omicron Variant (B.1.1.529 variant) was identified in South Africa in November of 2021. This variant has a large number of mutations that allow the virus to spread more easily and quickly than other variants. As of May 2022, Omicron variants have been divided into distinct sub-lineages: BA.1, BA.1.1, BA.2, BA.3, BA.2.12.1, BA.4, and BA.5. Among them, BA.4 and BA.5 have identical mutations on their spike protein. The spike proteins of BA.4 and BA.5 are referred to as BA.4/5 in this datasheet.
Vesicular stomatitis virus (VSV) is an enveloped, negative-stranded RNA virus that infects a wide range of animals and less frequently humans, causing mild flu-like symptoms. Its simple structure and its ability to grow in most mammalian cell types has made VSV a valuable tool to study virus entry, replication, and assembly. The glycoprotein of VSV (VSV-G), which binds to the LDL-receptor (low-density lipoprotein receptor), is responsible for the attachment and entry of VSV into a susceptible host cell. Recombinant VSV in which the glycoprotein was deleted (VSV Delta G) can accept viral envelop proteins from a variety of other viruses, allowing to generate pseudotypes that represent robust models to screen for neutralizing antibodies and other inhibitors of virus entry. The pseudoviruses can be engineered to transduce a reporter gene such as Firefly Luciferase or a fluorescent protein, so that viral entry can be monitored using luminescence or fluorescence.