Anti-CD19 CAR-T Cells
The anti-CD19 CAR-T cells are produced by high-titer lentiviral transduction of human primary CD4+CD8+ T cells using the anti-CD19 CAR Lentivirus (CD19 ScFv-CD8-4-1BB-CD3ζ; SIN Vector, BPS Bioscience #78601). These ready-to use CAR-T cells express an anti-CD19 CAR consisting of the ScFv (Single chain fragment variable) of anti-CD19 (clone FMC63) linked to a 2nd generation CAR (Chimeric Antigen Receptor) containing CD8 hinge and transmembrane domains, and the 4-1BB and CD3ζ signaling domains (Figure 1).
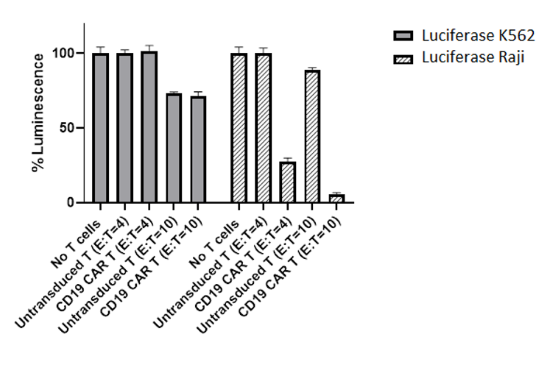
These CAR-T cells have been validated using flow cytometry (to determine the CAR expression) and co-culture cytotoxicity assays.

Figure 1: Construct diagram showing components of the anti-CD19 CAR expressed in anti-CD19 CAR-T cells.
| Name | Ordering Information |
| Human Interleukin-2 | BPS Bioscience #90184 |
| Human CD3/CD28/CD2 T Cell Activator | Stemcell technologies #10970 |
| CD19/Firefly Luciferase CHO Cell Line | BPS Bioscience #79714 |
| Firefly Luciferase CHO Cell Line | BPS Bioscience #79725 |
| Firefly Luciferase Raji Cell Line | BPS Bioscience #78622 |
| Firefly Luciferase K562 Cell Line | BPS Bioscience #78621 |
| Untransduced T Cells (Negative Control for CAR-T cells) | BPS Bioscience #78170 |
| ONE-Step™ Luciferase Assay System | BPS Bioscience #60690 |
| Luminometer |
Recommended anti-CD20 CAR-T Cell Medium: TCellM™ (BPS Bioscience #78753) supplemented with 10 ng/ml Interleukin-2 (BPS Bioscience #90184).
The cells have been screened to confirm the absence of Mycoplasma species.
CD19 (also known as Cluster of Differentiation 19, B-lymphocyte surface antigen B4, or CVID3) is a glycoprotein expressed at the surface of B lymphocytes through most phases of B cell maturation. It is strictly required for B cell terminal differentiation. Mutations in the CD19 gene cause severe immune-deficiency syndromes associated with impaired antibody production such as CVID3 (common variable immuno-deficiency 3). The majority of B cell malignancies express normal to high levels of CD19, which is a nearly ideal target for cancer immunotherapy. Blinatumomab, a CD19/CD3 bi-specific T cell engager (BiTE) has been approved for relapsed/refractory B-precursor ALL (Acute lymphoblastic leukemia). In addition, CD19 was the target of the first approved CAR-T cell therapy.