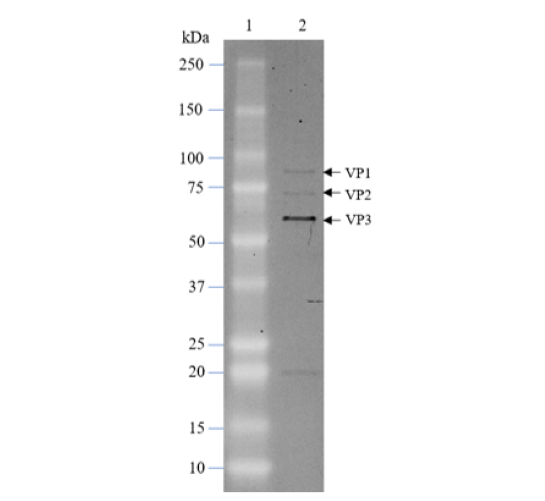
AAV-6 Anti-CD19 CAR (CD19 ScFv-CD8-4-1BB-CD3ζ)
The AAV-6 Anti-CD19 CAR (CD19 ScFv-CD8-4-1BB-CD3ζ) is an AAV 6 encoding the ScFv portion of anti-CD19 (clone FMC63) linked to the 2nd generation CAR (Chimeric Antigen Receptor) containing the CD8 hinge, 4-1BB and CD3ζ signaling domains, under the control of a EF1α promoter (Figure 1).

Figure 1. Schematic representation of AAV-6 Anti-CD19 CAR (CD19 ScFv-CD8-4-1BB-CD3ζ). The diagram indicates the components of the anti-CD19 CAR (ScFv-CD8-4-1BB-CD3ζ).
AAV was produced in HEK293 cells and is supplied in PBS-MK (PBS Magnesium-Potassium) buffer containing 0.01% Pluronic F68. Viral particles can be packaged in custom formulations by special request, for an additional fee.
Adeno-Associated Virus Serotype 6 (AAV6) appears to be related to AAV1 by sequence analysis. In addition to skeletal muscle cells, AAV6 is more efficient at transducing airway epithelial cells in mouse lungs than AAV2 and less immunogenic, which may make AAV6 more advantageous than AAV2 in gene therapy targeting lung diseases, like cystic fibrosis. It is also able to transduce pancreatic beta-cells more efficiently than AAV1. AAV6 is able to target hematopoietic lineage cells and have been used in dendritic cells and T and B cells.
CD19 (also known as Cluster of Differentiation 19, B-lymphocyte surface antigen B4, or CVID3) is a glycoprotein expressed at the surface of B lymphocytes through most phases of B cell maturation. It is strictly required for B cell terminal differentiation. Mutations in the CD19 gene cause severe immune-deficiency syndromes associated with impaired antibody production such as CVID3 (common variable immuno-deficiency 3). The majority of B cell malignancies express normal to high levels of CD19, making it a nearly ideal target for cancer immunotherapy. Blinatumomab, a CD19/CD3 bi-specific T cell engager (BiTE) has been approved for relapsed/refractory B precursor ALL (Acute lymphoblastic leukemia) and CD19 was the target of the first approved CAR-T cell therapy. Studies of CD19 function and expression profiles will continue to broaden our knowledge and support broader applications in cancer therapy.