Anti-CD19 CAR Negative Control/NFAT (Luciferase) Reporter Jurkat Cell Line (CD19 SCFV-CD28 Transmembrane Motif)
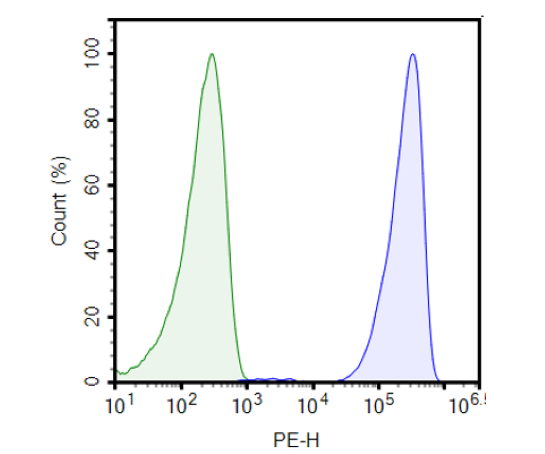
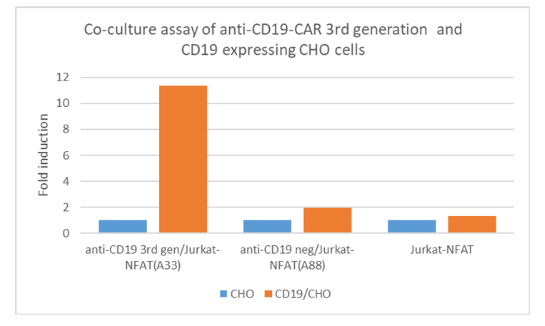
Anti-CD19 CAR Negative Control/NFAT (Luciferase) Reporter Jurkat Cell Line (CD19 SCFV-CD28 Transmembrane Motif) is a stable cell line expressing an anti-CD19 CAR negative control and an NFAT-dependent luciferase reporter. The anti-CD19 CAR negative control consists of anti-CD19 scFv linked to the CD28 transmembrane motif without the intracellular signaling domains. The reporter cell line has been validated for anti-CD19 expression by flow cytometry, and in co-culture with target cells, such as CD19/CHO recombinant cell line the luciferase reporter gene was not activated. Anti-CD19 CAR Negative Control Jurkat/NFAT (Luciferase) Reporter Cell Line was generated by the transduction of NFAT Luciferase Reporter Jurkat Cell Line (BPS Bioscience #60621) with anti-CD19 CAR negative control lentivirus.
The cell line can be used as negative control for the Anti-CD19 CAR/ NFAT (Luciferase) Reporter Jurkat Cell Line (CD19 SCFV-CD28-4-1-BB-CD3ζ) (BPS Bioscience #79853).

Figure 1: Lenti-vector used to generate anti-CD19 CAR negative control lentivirus.
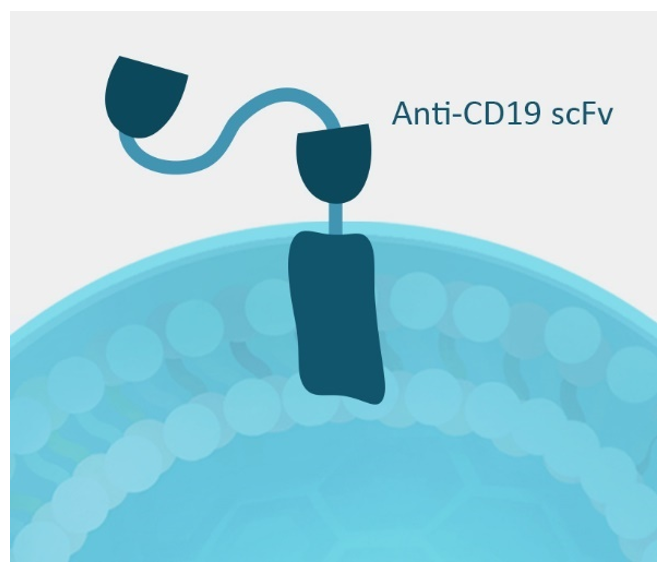
Figure 2. Schematic of anti-CD19 CAR negative control.
The anti-CD19 (scFv) is linked to the CD28 transmembrane motif.
Purchase of this cell line is for research purposes only; commercial use requires a separate license. View the full terms and conditions.
Media Required for Cell Culture
| Name | Ordering Information |
| Thaw Medium 2 | BPS Bioscience #60184 |
| Growth Medium 2H | BPS Bioscience #79784 |
Materials Required for Cellular Assay
| Name | Ordering Information |
| Thaw Medium 2 | BPS Bioscience #60184 |
| Thaw Medium 3 | BPS Bioscience #60186 |
| CD19, Fc-Fusion (IgG1), Avi-Tag, Biotin labeled | BPS Bioscience #79475 |
| CD19 CHO Recombinant Cell Line | BPS Bioscience #79561 |
| NFAT Reporter (Luc)- Jurkat Recombinant Cell Line | BPS Bioscience #60621 |
| Anti-CD19 CAR / NFAT (Luciferase) Reporter Jurkat Cell Line (CD19 SCFV-CD28-4-1BB-CD3ζ) | BPS Bioscience #79853 |
| Empty vector control – CHO-K1 Recombinant Cell line | BPS Bioscience #60545 |
| PE Streptavidin | Biolegend #405203 |
| 7-AAD | BioLegend #420403 |
| ONE-Step™ Luciferase Assay System | BPS Bioscience #60690 |
| Luminometer |
The cell line has been screened to confirm the absence of Mycoplasma species.
CD19 (also known as Cluster of Differentiation 19, B-lymphocyte surface antigen B4, or CVID3) is a glycoprotein expressed at the surface of B lymphocytes through most phases of B cell maturation. It is strictly required for B cell terminal differentiation. Mutations in the CD19 gene cause severe immune-deficiency syndromes associated with impaired antibody production, such as CVID3 (common variable immuno-deficiency 3). The majority of B cell malignancies express normal to high levels of CD19, making it a nearly ideal target for cancer immunotherapy. Blinatumomab, a CD19/CD3 bi-specific T cell engager (BiTE) has been approved for relapsed/refractory B precursor ALL (Acute lymphoblastic leukemia) and CD19 was the target of the first approved CAR-T cell therapy. Studies of CD19 function and expression profiles will continue to broaden our knowledge and support further applications in cancer therapy.
- Wang H, et al., 2019 J Hematol Oncol. 12(1):59-78.
- Hasegawa K. and Hosen N., 2019 Inflamm Regen. 39:10-14.
- Giuliani N, et al., 2019 Expert Rev Hematol. 12(7):481-496.