CAR T-Cell Therapy
Overview
CAR-T is a cross of immunotherapy, gene therapy, and cellular therapy. Many successful immunotherapies are based on checkpoint inhibitors that block the mechanisms tumor cells use to hide from T cells. CAR-T immunotherapies go one step further by engineering T cells to enhance the immune response against a specific tumor antigen. CAR-T is a promising approach for cancer and other diseases, especially severe cancers that do not respond well to other treatments. Huge remission rates of up to 94% are observed in clinical trials of CAR-T, and two therapies are already FDA approved (Novartis, Gilead). These dramatic improvements in patient health have led to over 240 CAR-T clinical trials1, and advances in CAR-T and adoptive cell transfer therapies are expected to continue to accelerate as researchers develop a better understanding of how these therapies work in patients. CAR stands for Chimeric Antigen Receptor. CARs are synthetic receptors built to recognize a particular cancer antigen. The CAR is cloned and expressed in T cells removed from the patient. These cells are tested to ensure they are expressing the CAR, and the resulting CAR T cells are infused back into the patient to directly target antigen-expressing cancer cells. Both of the two FDA-approved CAR-T therapies target CD19.
CAR-T and Cytotoxicity
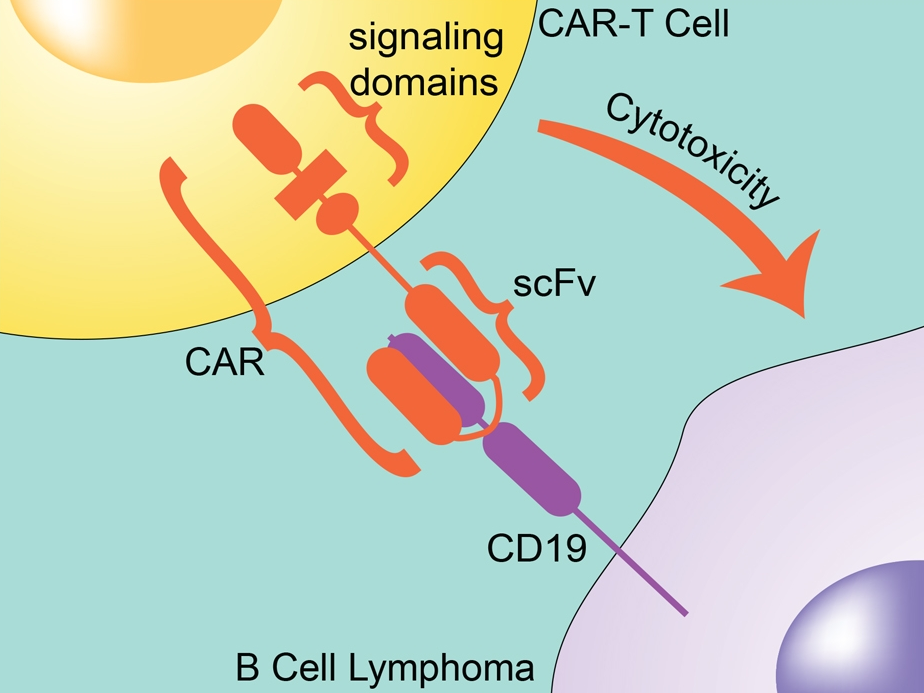
CARs are designed to include the single-chain variable fragment (scFV) of a monoclonal antibody. They also contain intracellular signaling domains, such as the T-cell receptor CD3ζ chain, that mediates T-cell activation, cytokine release, and cytotoxicity when bound to a target cell. Since the CAR already includes the CD3 chain, the T cell doesn’t require the MHC/TCR step for activation. The CAR not only directs the T cell to the cancer antigen, it also activates the T cell to kill it.
Unfortunately, there can be serious side effects from CAR-T, including death. The worst cases result from uncontrolled cytokine release in what is known as cytokine release syndrome. Much of the research on second generation CAR-T is directed toward minimizing these potential side effects. Additional regulation comes by adding a second signaling domain such as CD28, associated with the clonal expansion of T cells, or 4-1BB (CD137), associated with longer duration in circulation.
Signaling Domains
Third generation CAR improvements include signaling domains such as CD27, CD28, ICOS, and OX40, which improve targeting of the CAR-T cell. Not surprisingly, many of these extra signaling domains are derived from important checkpoint inhibitor pathways. Some of the clinical trials are investigating combination therapies by including CAR-T with chemotherapy or with checkpoint inhibitors (immunotherapy). Also, since CAR-T therapy is not MHC-dependent, other researchers are developing “off the shelf” CAR-T from healthy donors that can be used in any patient whose cancer is expressing that particular cancer antigen recognized by the CAR-T.
Evaluating CAR expression is essential for the production of CAR-T cells. BPS has created cell lines expressing important cancer antigens, enabling researchers to demonstrate that their CAR-T cells are specific for particular targets. Beyond CD19, these cell lines express BCMA, CD47, ERBB2, CD20, PD-L1, CD22, ROR1, CD37, SLAMF7, and CD38.
Cancer Antigens
Cancer cells express many different kinds of antigens, including tumor-specific antigens (TSA), unique to tumor cells, and tumor-associated antigens (TAA) which are more general. Importantly, the expression level of these antigens can change significantly over time, depending on the type of the tumor and the stage of the cancer.
Expression levels also correlate with tumor progression and prognosis, and the level of certain cancer antigens can be used to monitor the success of therapeutic treatments.). TAAs are normally expressed only in certain cell types or during certain states of development.
Many cancer cells also express mutated versions of normal proteins associated with growth, proliferation, and differentiation, which allows them to rapidly multiply and metastasize.
Products
BPS has developed stable, recombinant cell lines expressing cancer antigens at different expression levels--high, medium, or low expression. These cell lines are ideal to monitor the effectiveness of different CAR-T at detecting the target antigen expressed at different levels, which may relate to the time dependence of antigen expression. The FACS data analysis to the right shows several BCMA-expressing cell lines detected by a PE-labeled antibody, vs. the control cell line.
BPS also offers biotin-labeled versions of many of the antigens recognized by CAR-T cells, such as BCMA, CD19, CD22, CD123, CD38, and ROR1. These biotin-labeled proteins can bind to CAR-T cells and be detected by flow cytometry using PE-streptavidin, in order to verify that the CAR-T cell is recognizing and binding the specific cancer antigen.
Engineering T cells to boost the immune system has potential benefits beyond cancer treatment, including treatments for chronic inflammatory diseases such as hepatitis, HIV/AIDS, lupus, and arthritis. Companies are also investigating CAR T-cell therapy in organ transplantation to eliminate the need for lifelong immunosuppressants. The future is bright for CAR-T research, and BPS Bioscience continues to develop unique cell lines and other tools to help researchers create, evaluate, and enhance CAR-T cells for the improvement of human health.
References:
- Sadelain, M. 2017. CD19 CAR T Cells. Cell. 2017 Dec 14;171(7):1471. doi: 10.1016/j.cell.2017.12.002.
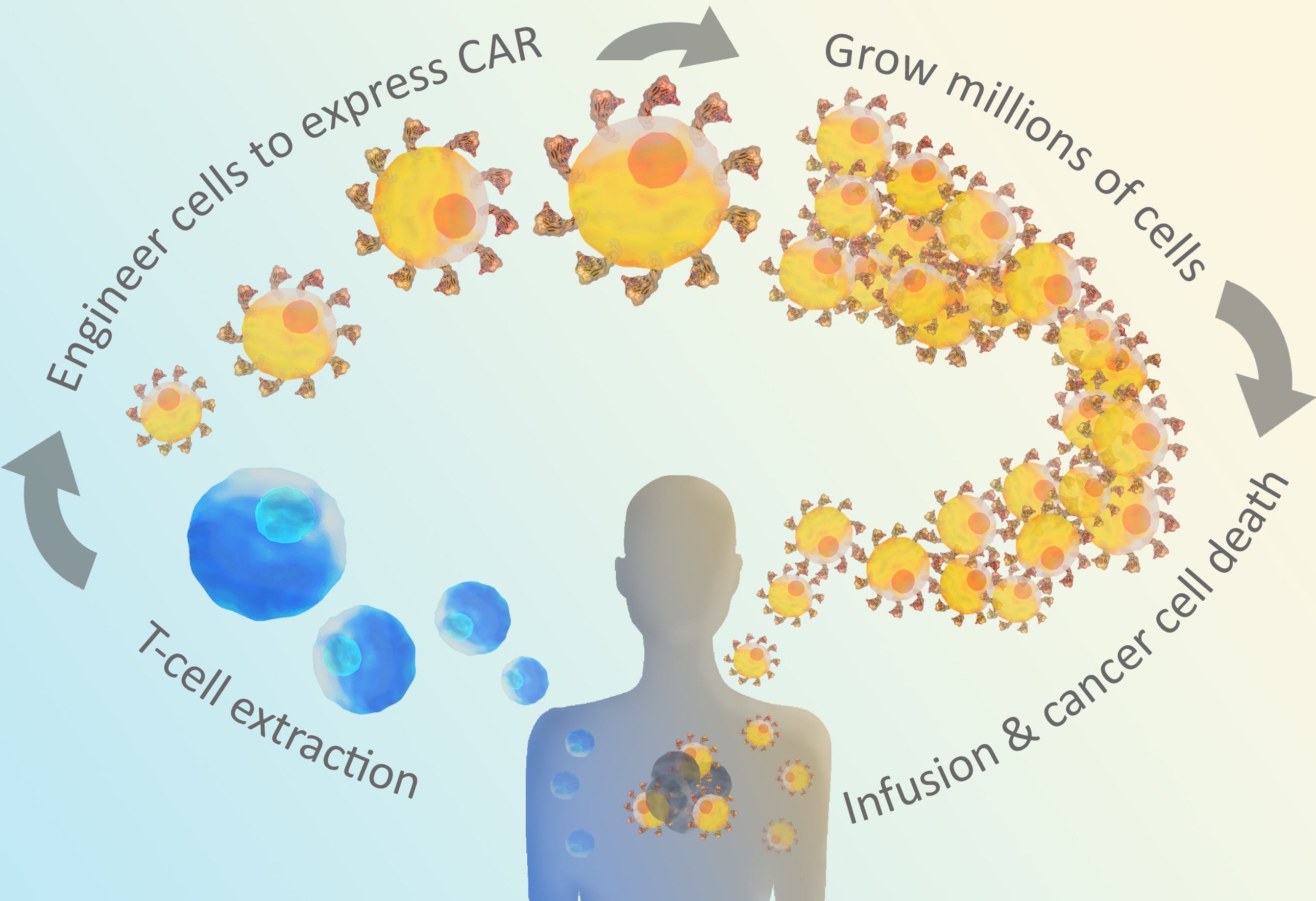
Figure 1: CAR T-cells are engineered through extracted t-cells from the patient.
Figure 2: CAR T-cell with the single-chain variable fragment (scFV) of a monoclonal antibody.
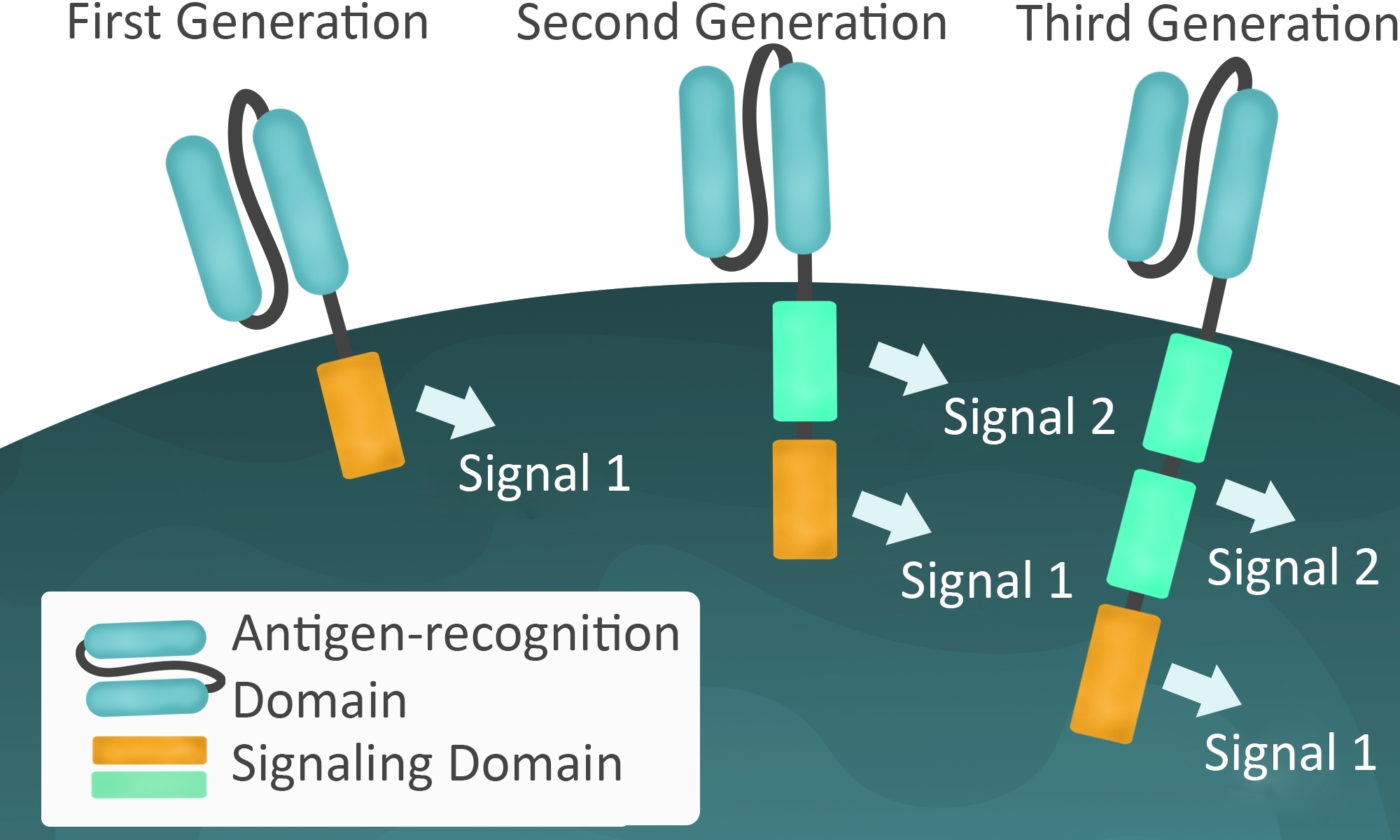
Figure 3: First, second, and third generation signaling domains
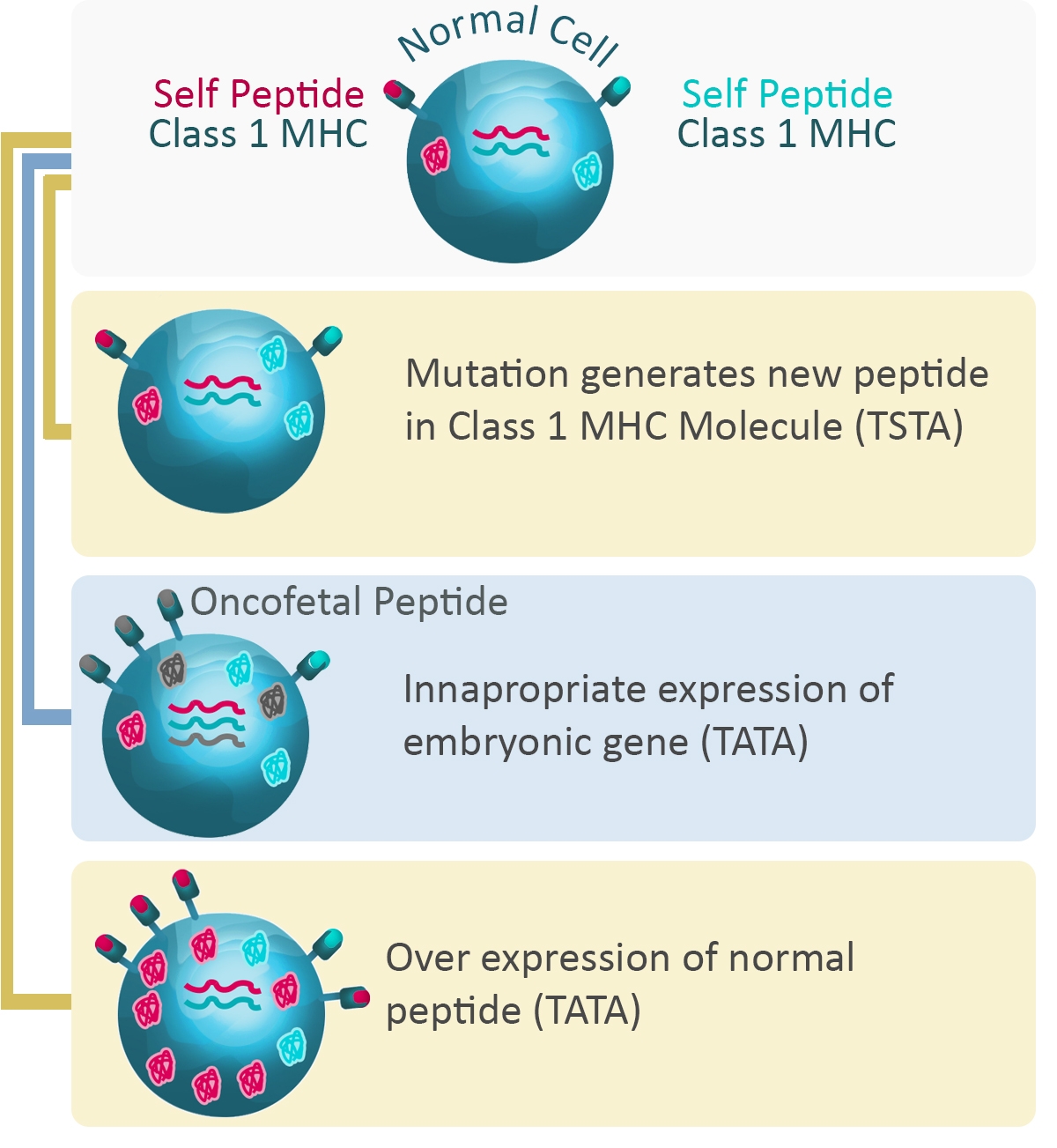
Figure 4: Expression of proteins and cancer
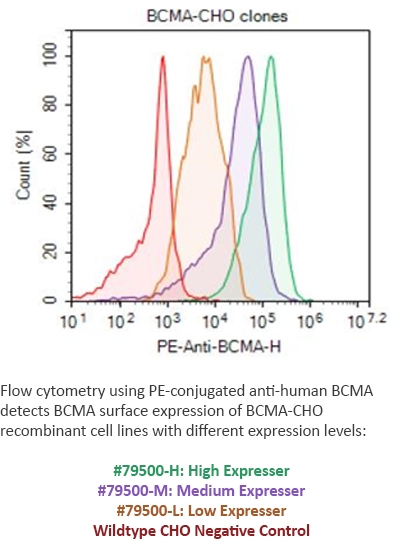
Figure 5: FACS from BCMA CHO Cell Line