BCMA / CD20 / Firefly Luciferase CHO Cell Line
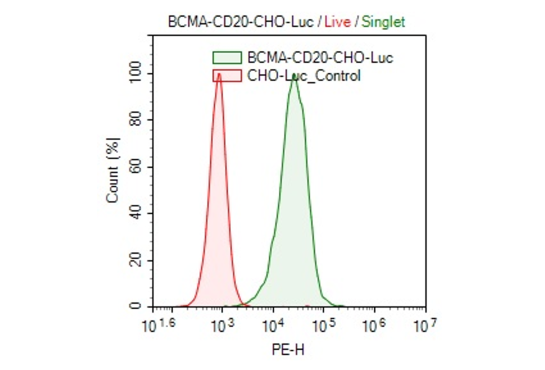
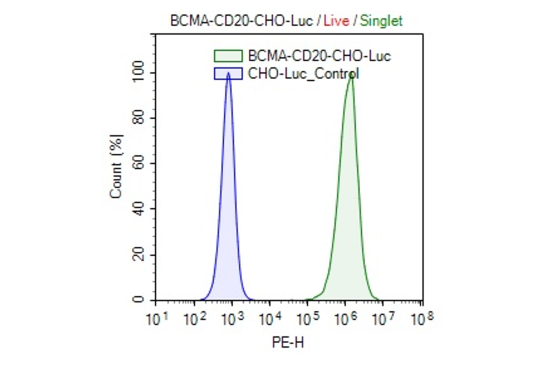
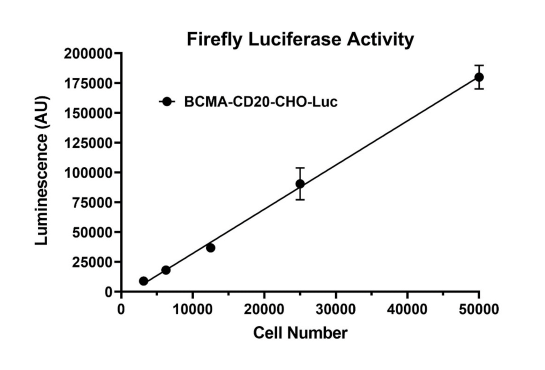
Clonal stable CHO cell line constitutively expressing full length human BCMA protein (also known as CD269 or TNFRSF17, Genbank accession #NM_001192) and human CD20 protein (also known as MS4A1 or FMC7, Genbank accession #NM_021950). This cell line was derived from our CHO-K1 Luciferase cells (BPS Bioscience, #79725), therefore it also constitutively expresses the firefly luciferase reporter. Surface expressions of BCMA and CD20 were confirmed by flow cytometry.
Purchase of this cell line is for research purposes only; commercial use requires a separate license. View the full terms and conditions.
Materials Required for Cell Culture
| Name | Ordering Information |
| Thaw Medium 3 | BPS Bioscience #60186 |
| Growth Medium 3K | BPS Bioscience #78041 |
Materials Required for Cellular Assay
| Name | Ordering Information |
| ONE-Step™ Luciferase Assay System | BPS Bioscience #60690 |
| 96-well tissue culture-treated white clear-bottom assay plate |
|
| Luminometer |
The cell line has been screened using the MycoAlert™ Mycoplasma Detection kit (Lonza, #LT07-218) to confirm the absence of Mycoplasma species.
B-Cell Maturation Antigen (BCMA), also known as CD269, is a cell surface receptor of the TNF receptor superfamily that recognizes B-Cell Activating Factor (BAFF). BCMA is preferentially expressed on mature B-lymphocytes and Multiple Myeloma (MM) cells. BCMA is a highly attractive target antigen for immunotherapy, not only because of its restricted expression in nonmalignant tissue, but also due to its almost universal expression on MM cells. Pre-clinical studies using CAR (Chimeric Antigen Receptor) T-cells targeting BCMA have demonstrated anti-MM activity, and in 2017, the FDA granted BCMA CAR T-Cell immunotherapy the breakthrough designation in treating Multiple Myeloma.
CD20 (MS4A1) is a glycosylated phosphoprotein expressed on the cell surface of B cells. Although the functional significance of CD20 is not clear, and CD20 has no known ligands, CD20 has been shown to regulate intracellular calcium levels. CD20 is a highly attractive target antigen for immunotherapy because it is expressed in more than 90% of patients with B-cell lymphoma. First approved in 1997, Rituximab (Rituxan) is a chimeric monoclonal antibody targeting CD20 and has been classified by the World Health Organization as an “Essential Medicine”. Since then, additional monoclonal antibodies against CD20 have been approved or are being tested in clinical trials for the treatment of multiple sclerosis (MS), chronic lymphocytic leukemia (CLL), follicular lymphoma, diffuse large B cell lymphoma (DLBCL), rheumatoid arthritis, non-Hodgkin’s lymphoma, systemic lupus erythematosus, and myalgic encephalomyelitis (chronic fatigue syndrome). Additionally, more recently, anti-CD20-CD19 bispecific CAR-T cells have been developed to address concerns over potential relapse.