BCMA / GLuc - CHO Recombinant Cell Line
Catalog #
79830
$7,150
*
●
●
Purchase
Description
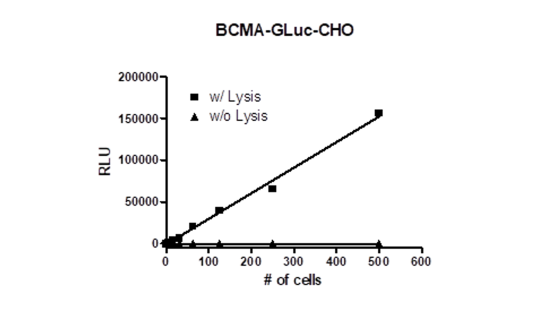
Recombinant CHO-K1 cells constitutively expressing both the human BCMA protein (B-Cell Maturation Antigen or CD269, GenBank accession #NM_001192) and the Gaussia Luciferase (Δ Signal peptide). Surface expression of BCMA was confirmed by flow cytometry.
Purchase of this cell line is for research purposes only; commercial use requires a separate license. View the full terms and conditions.
●
Synonyms
CHO-K1 cell lne, CD269 cell line
●
Product Data Gallery
Product Info
Storage and Usage
Citations
Host Cell Line
CHO-K1
Supplied As
Each vial contains 2 x 106 cells in 1 ml of FBS with 10% DMSO.
Materials Required But Not Supplied
• Thaw Medium 3 (BPS Bioscience, #60186)
• Growth Medium 3F (BPS Bioscience, #79829)
• 96-well tissue culture-treated white clear-bottom assay plate
• Cell lysis buffer
• Coelenterazine (NanoLight, #303)
• Luminometer
UniProt #
Q02223
Mycoplasma Testing
This cell line has been screened using the MycoAlert™ Mycoplasma Detection Kit (Lonza,
#LT07-118) to confirm the absence of Mycoplasma contamination. MycoAlert Assay Control Set
(Lonza, #LT07-518) was used as a positive control.
Background
B-Cell Maturation Antigen (BCMA), also known as CD269, is a cell surface receptor of the TNF receptor superfamily that recognizes B-Cell Activating Factor (BAFF). BCMA is preferentially expressed on mature B-lymphocytes and Multiple Myeloma (MM) cells. BCMA is a highly attractive target antigen for immunotherapy, not only because of its restricted expression in non-malignant tissue, but also due to its almost universal expression on MM cells. Pre-clinical studies using CAR (Chimeric Antigen Receptor) T-cells targeting BCMA have demonstrated anti-MM activity, and in 2017, the FDA granted BCMA CAR T-Cell immunotherapy the breakthrough designation for treating Multiple Myeloma.