Anti-BCMA CAR /NFAT (Luciferase) Reporter Jurkat Cell Line
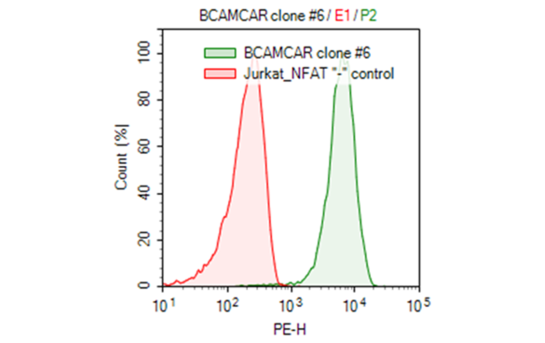
The anti-BCMA CAR /NFAT (luciferase) reporter Jurkat cell line is a stable cell line made from the anti-BCMA scFV CAR lentivirus (BPS Bioscience #79701). It has been validated for anti BCMA-CAR expression by FACS, and for functional activation stimulated by both soluble BCMA protein (BPS Bioscience #79467) and BCMA/CHO target cells (BPS Bioscience #79500).
Interested in screening and profiling anti-BCMA CAR cells in your cell models without the need to purchase and license the cell line? Check out our Immunotherapy Cell-Based Screening Services.
Purchase of this cell line is for research purposes only; commercial use requires a separate license. View the full terms and conditions.
• CHO-K1 cell line (ATCC) and target cell BCAM/CHO stable cell line (BPS Bioscience #79500)
• Thaw Medium 3 (BPS Bioscience #60186): Ham’s F-12 medium (Hyclone #SH30526.01) supplemented with 10% FBS (Thermo Fisher, #26140079), 1%
Penicillin/Streptomycin (Hyclone SV30010.01).
• Growth Medium 3D (BPS Bioscience #79539): Thaw Medium 3 plus 1 mg/ml Geneticin (Thermo Fisher, #11811031).
• NFAT(luciferase) reporter Jurkat cell line (BPS Bioscience #60621)
• Thaw Medium 2 (BPS Bioscience #60184): RPMI 1640 medium (Thermo Fisher,
#A1049101) supplemented with 10% FBS (Thermo Fisher, #26140079), 1%
Penicillin/Streptomycin (Hyclone #SV30010.01).
• Growth Medium 2H (BPS Bioscience #79784): Thaw Medium 2 plus 1 μg/ml puromycin
(InvivoGen # ant-pr-1) and 1 mg/ml of Geneticin (Thermo Fisher, #11811031).
• 96-well tissue culture treated white clear-bottom assay plate (Corning #3610)
• One-Step luciferase assay system (BPS Bioscience #60690)
• Luminometer
The development of CAR-T cells is a complex process that requires I) screening and sequencing of mAbs that are specific to the cancer antigens; II) synthesis of scFv cDNA and clone into Chimeric Antigen Receptor (CAR) cassette in Lentivector (e.g. anti-BCMA scFv in 3rd generation CAR cassette in lentivector); III) packaging and production of high titer lentivirus CAR encoding lentivirus; IV) isolation, activation and expansion of patient-derived T cells that exhibit a specific cellular phenotype (e.g. CD4+ or CD8+ or a mix); V) and transduction of activated T cells with CAR-encoding lentivirus; VI) Validation of engineered CAR-T cells through FACS and functional analysis. BPS has developed an anti-BCMA CAR /NFAT(luciferase) reporter Jurkat stable cell line, it is one of a series of reporter bioassays using CAR-T Lentivirus and /NFAT(luciferase) reporter Jurkat cell lines. The anti-BCMA CAR /NFAT(luciferase) reporter Jurkat cell line is a great system to predict the mechanism of action (MOA) and therapeutic potential of the anti-BCMA CAR lentivirus before using it with patient-derived primary T cells. It is a single cell clonal stable cell line developed by transducing the Jurkat/NFAT-Luciferase reporter cells with the anti-BCMA scFV CAR lentivirus (BPS Bioscience #79701).
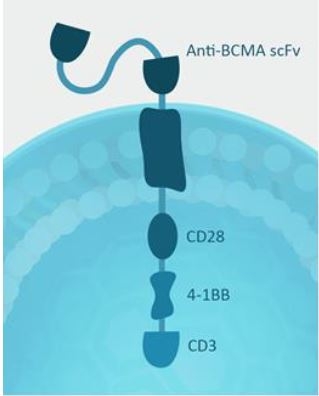
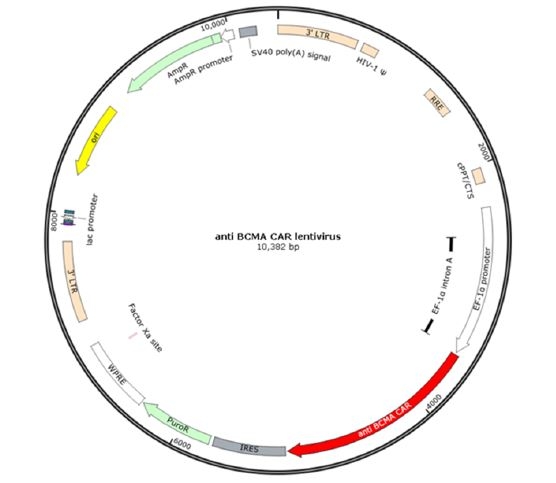
Figure 1. Anti-BCMA CAR design and lentivector:
Figure 2. Schematic of the lenti-vector used to generate the anti-BCMA CAR/ NFAT (luciferase) Jurkat stable cell line.
The anti-BCMA (scFv) is linked to the 3rd generation CAR with CD28 transmembrane and costimulatory domains, 4-1BB, and CD3ζ components:
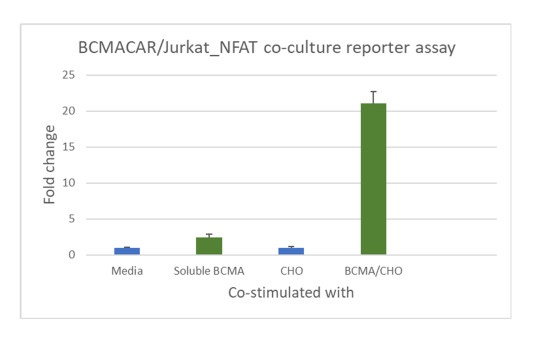
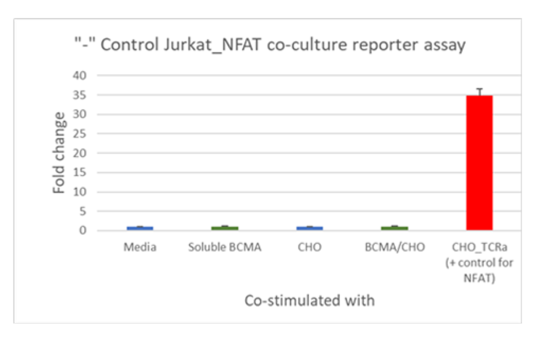
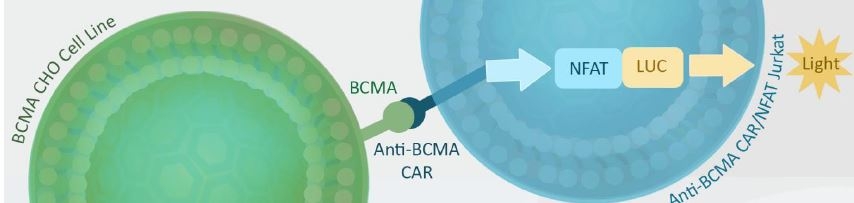
Figure 3. Functional luciferase assay to test the specificity and potency of anti-BCMA scFv CAR/ NFAT (luciferase) reporter Jurkat cell line using BCMA/CHO target cells co-culture:
1. Immune checkpoint blockade and CAR-T cell therapy in hematologic malignancies. Wang et. al. J Hematol Oncol. 2019 Jun 11;12(1):59
2. Chimeric antigen receptor T cell therapy for multiple myeloma. Hasegawa et.al. Inflamm Regen. 2019 Jun 4;39:10.
3. Novel targets for the treatment of relapsing multiple myeloma. Giuliani et. al. Expert Rev Hematol. 2019 Jun 3:1-16.
4. Anti-BCMA antibodies in the future management of multiple myeloma. Gavriatopoulou et. al. Expert Rev Anticancer Ther. 2019 Apr;19(4):319-326.