Anti-CD20 CAR-T Cells
The anti-CD20 CAR-T cells are produced by high-titer lentiviral transduction of human primary CD4+CD8+ T cells using the anti-CD20 CAR Lentivirus (CD20 ScFv-CD8-4-1BB-CD3ζ; BPS Bioscience #78606). These ready-to use CAR-T cells express an anti-CD20 CAR consisting of the ScFv (Single chain fragment variable) of anti-CD20 (clone Leu-16) linked to a 2nd generation CAR (Chimeric Antigen Receptor) containing CD8 hinge and transmembrane domains, and the 4-1BB and CD3ζ signaling domains (Figure 1).
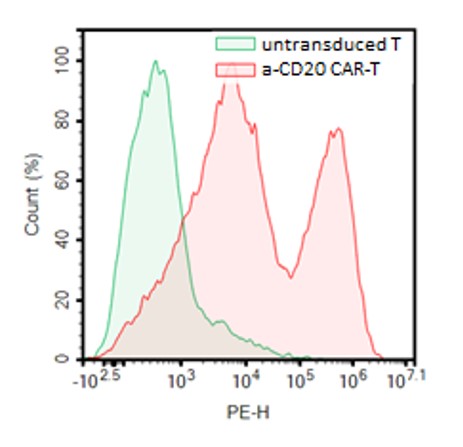
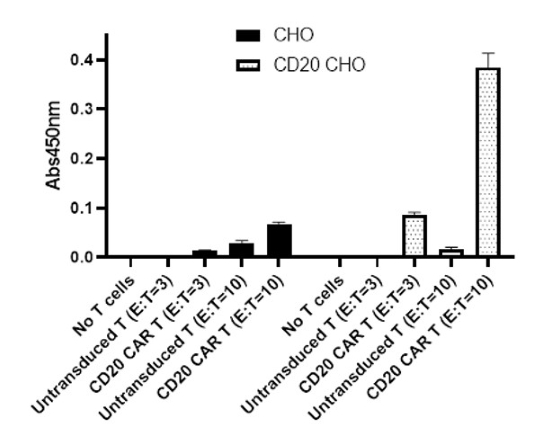
These CAR-T cells have been validated using flow cytometry (to determine the CAR expression) and co-culture cytotoxicity assays.
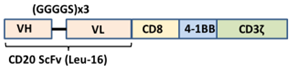
Figure 1: Construct diagram showing components of the anti-CD20 CAR expressed in anti-CD20 CAR-T cells.
| Name | Ordering Information |
| Human Interleukin-2 | BPS Bioscience #90184 |
| Human CD3/CD28/CD2 T Cell Activator | Stemcell technologies #10970 |
| Biotinylated Protein L | Genscript #M00097 |
| PE-Streptavidin | Biolegend #405203 |
| CD20 CHO Recombinant Cell Line (High Expression) | BPS Bioscience #79624-H |
| IFN-g (Human) Colorimetric ELISA Detection Kit | BPS Bioscience #79777 |
Recommended anti-CD20 CAR-T Cell Medium: TCellM™ (BPS Bioscience #78753) supplemented with 10 ng/ml Interleukin-2 (BPS Bioscience #90184).
The cells have been screened to confirm the absence of Mycoplasma species.
CD20 (also known as MS4A1) is a glycosylated phosphoprotein expressed on the cell surface of B cells. CD20 is a highly attractive target antigen for immunotherapy because it is highly expressed in more than 90% of patients with B-cell lymphoma. First approved in 1997, Rituximab (Rituxan) is a chimeric monoclonal antibody targeting CD20 and has been classified by the World Health Organization as an “Essential Medicine”. Since then, additional monoclonal antibodies against CD20 have been approved or are being tested in clinical trials for the treatment of multiple sclerosis (MS), chronic lymphocytic leukemia (CLL), follicular lymphoma, diffuse large B cell lymphoma (DLBCL), rheumatoid arthritis, non-Hodgkin’s lymphoma, systemic lupus erythematosus, and myalgic encephalomyelitis (chronic fatigue syndrome). More recently, anti-CD20-CD19 bispecific CAR-T cells have been developed to address concerns over potential relapse in cancer patients.