CD20 CHO Recombinant Cell Line (High or Medium Expression)
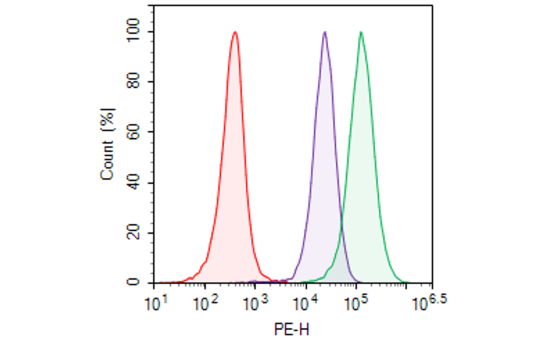
Recombinant clonal stable CHO cell line constitutively expressing full length human CD20 protein, also known as MS4A1 (Genbank #NM_152866). Surface expression of CD20 was confirmed by flow cytometry. Each stable clonal cell line was selected for different levels of CD20 expression (High, Medium) to mimic different stages of cancer target cells with various CD20 expression levels.
Purchase of this cell line is for research purposes only; commercial use requires a separate license. View the full terms and conditions.
CD20 (MS4A1) is a glycosylated phosphoprotein expressed on the cell surface of B cells. Although the functional significance of CD20 is not clear, and CD20 has no known ligands, CD20 has been shown to regulate intracellular calcium levels. CD20 is a highly attractive target antigen for immunotherapy because it is expressed on more than 90% of patients with B-cell lymphoma. First approved in 1997, Rituximab (Rituxan) is a chimeric monoclonal antibody targeting CD20 and has been classified by the World Health Organization as an “Essential Medicine”.
Since then, additional monoclonal antibodies against CD20 have been approved or are being tested in clinical trials for the treatment of multiple sclerosis (MS), chronic lymphocytic leukemia (CLL), follicular lymphoma, diffuse large B cell lymphoma (DLBCL), rheumatoid arthritis, non-Hodgkin’s lymphoma, systemic lupus erythematosus, and myalgic encephalomyelitis (chronic fatigue syndrome). Additionally, more recently, anti-CD20-CD19 bispecific CAR-T cells have been developed to address concerns over potential relapse.