Methods For Drug Discovery
Immunotherapies based on targeting the PD-1:PD-L1 immune checkpoint pathway are having clinical impact and are giving new hope to cancer patients. However, positive response to treatment is not guaranteed and resistance remains a concern. Improving outcomes based on immunotherapy requires multiple approaches for manipulating the complexities of the immune system and tumor microenvironment. To meet these challenges, a series of research tools have been created for screening antibodies, biologics, and small molecules against multiple immune-checkpoint pathways. Targets have been chosen from both immune activators and suppressors with the aim of obtaining precise control of the immune system.
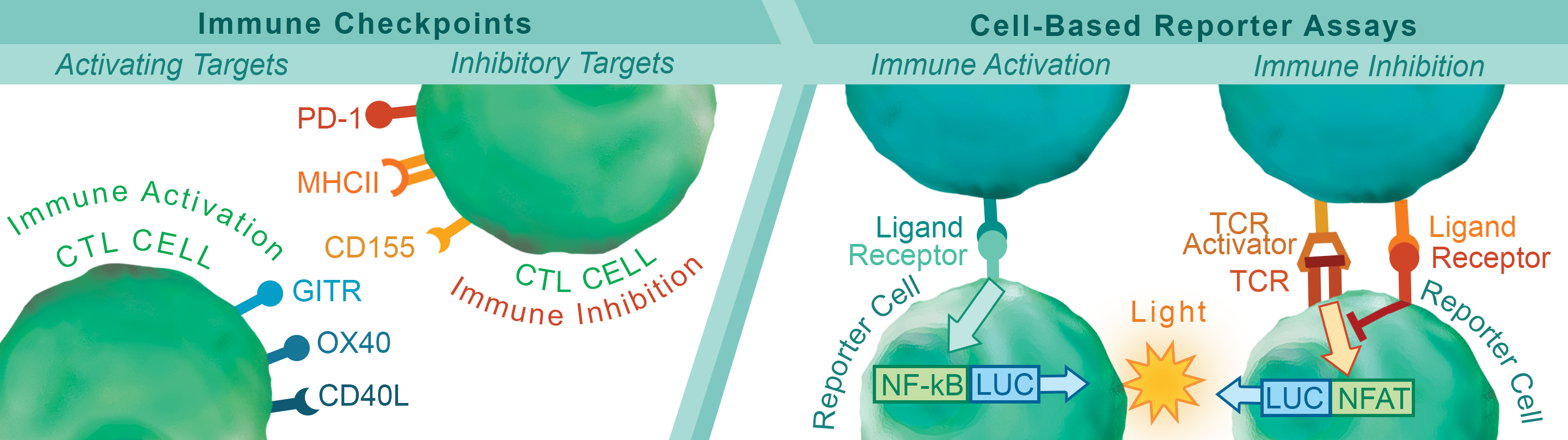
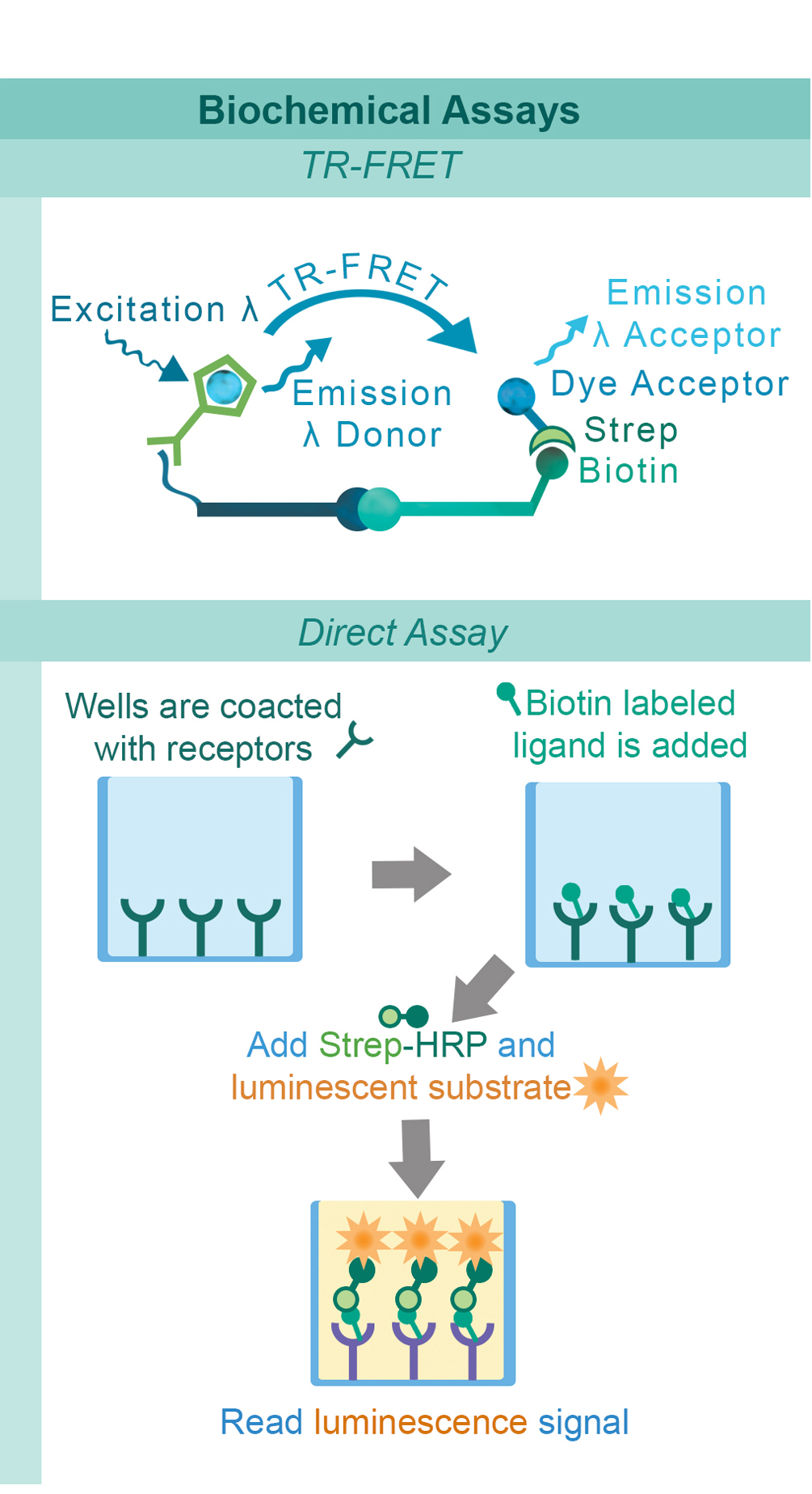
Biochemical assays have been developed to identify drug candidates that can modify the ligand-receptor interactions of these pathways in a high-throughput setting. To complement these biochemical assays, we have also created quantitative cell-based reporter assays. These reporter assays provide the means to investigate immune checkpoints in a cellular environment without the limitations and variability associated with assays based on primary donor cells. This combination of complementary techniques provides researchers with a comprehensive approach for identifying and developing the next generation of immunotherapy treatments.
The search for effective co-immunotherapies has expanded beyond PD-1 to several other checkpoint receptors. The availability of engineered cell-based assays simplifies and accelerates this research. Convenient reagents such as the One Step Luciferase Assay System (#60690) complement these cell systems by providing validated, consistent results. Finally, biochemical assay kits provide reliable, orthogonal assays for validating cell-based results and deeper mechanistic studies.
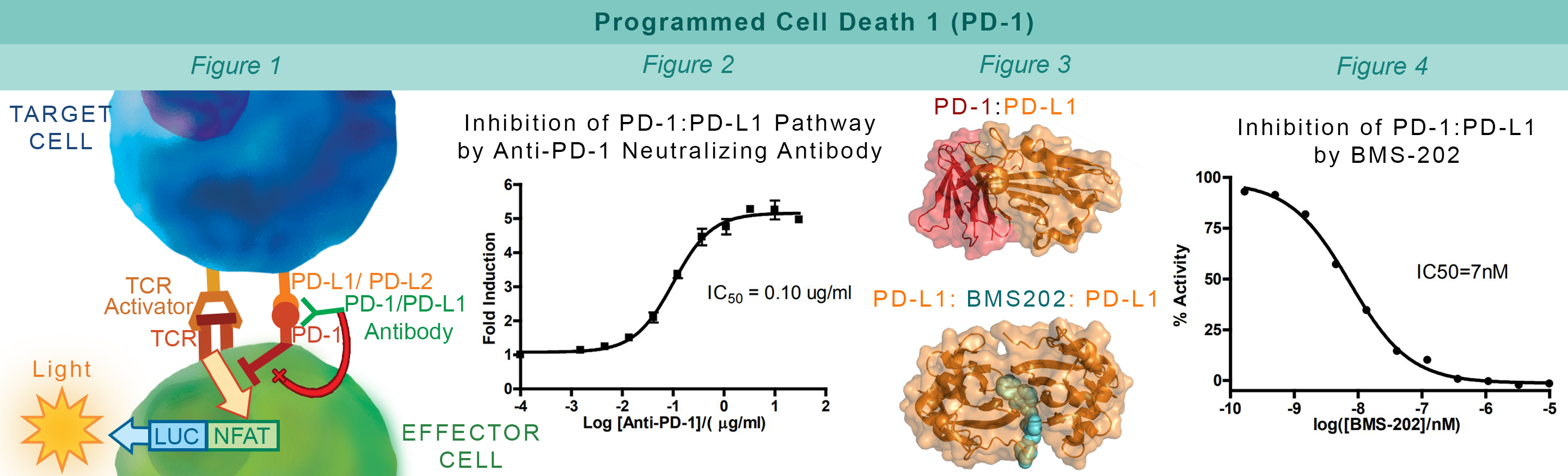
PD-1, a receptor expressed on activated T cells, binds to its ligand PD-L1 and negatively regulates immune responses. PD-1 ligands are found on most cancer cells, and the PD-1:PD-L1 interaction allows cancer cells to escape immune surveillance. Jurkat cells constitutively expressing PD-1 and the firefly luciferase gene controlled by NFAT response elements interact with cells constitutively expressing PD-L1 and an engineered TCR-activator (Figure 1). The PD-1:PD-L1 interaction between these cells prevents luciferase expression. The anti-PD-1 antibody inhibits PD-1:PD-L1 interaction allowing luciferase expression and production of reporter signal (Figure 2). The in vitro biochemical assay of protein-protein interaction between the extracellular domains of PD-1 and PD-L1 is monitored by TR-FRET. BMS202 inhibits the PD-1:PD-L1 interaction by stabilizing PD-L1:PD-L1 dimerization (Figure 3 & 4).
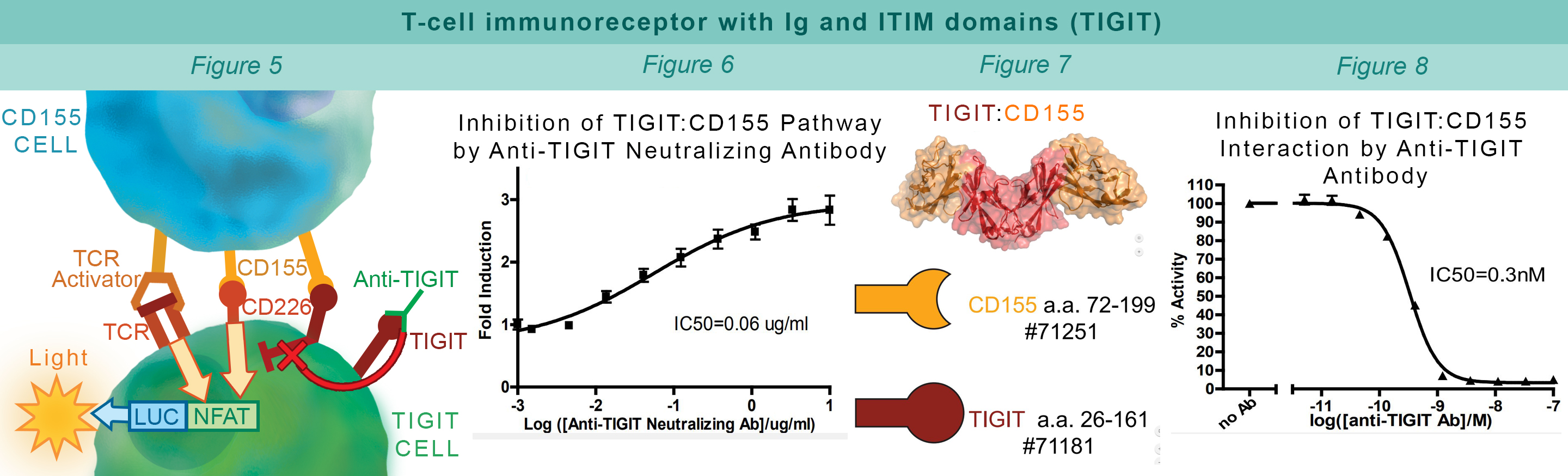
TIGIT is a co-inhibitory receptor expressed in Natural killer cells and activated regulatory T cells. Interaction with CD155 on antigen presenting cells suppresses NF-κB and NFAT-TCR signaling. This blocks T cell proliferation and cytokine production. CHO-K1 cells constitutively expressing CD155 and a TCR-activator (#60548) interact with Jurkat cells that express the firefly luciferase gene controlled by NFAT response elements and constitutively express TIGIT. The TIGIT:CD155 interaction inhibits luciferase expression (#60538) (Figure 5). Anti-TIGIT antibody inhibits the TIGIT:CD155 interaction allowing production of reporter signal (Figure 6). The AlphaLISA® proximity assay is used to monitor the protein-protein interaction between the purified extracellular domains of TIGIT:CD155 (#72029) (Figure 7). The Anti-TIGIT antibody inhibits the TIGIT:CD155 interaction (Figure 8).
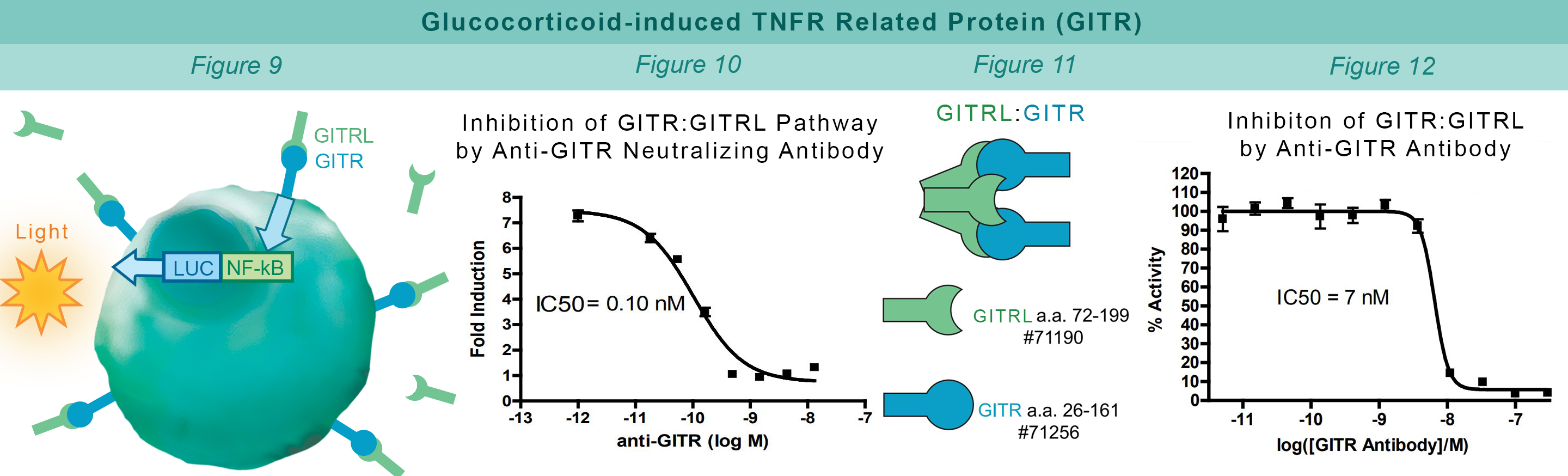
GITR is expressed in a number of cells including T cells, natural killer cells and antigen-presenting cells (APC’s). GITRL is expressed mainly by APCs and the GITR:GITRL interaction contributes to activation of the immune system. Jurkat cells constitutively expressing GITR and a luciferase gene controlled by NF-κB response elements are treated with soluble GITRL protein (#71190). The GITR:GITRL interaction stimulates luciferase expression (Figure 9). The anti-GITR antibody inhibits GITR:GITRL interaction preventing production of reporter signal (Figure 10). The TR-FRET proximity assay is used to monitor the protein-protein interaction between the purified, extracellular domains of GITR and GITRL (Figure 11). The anti-GITR antibody inhibits the GITR:GITRL interaction (Figure 12).
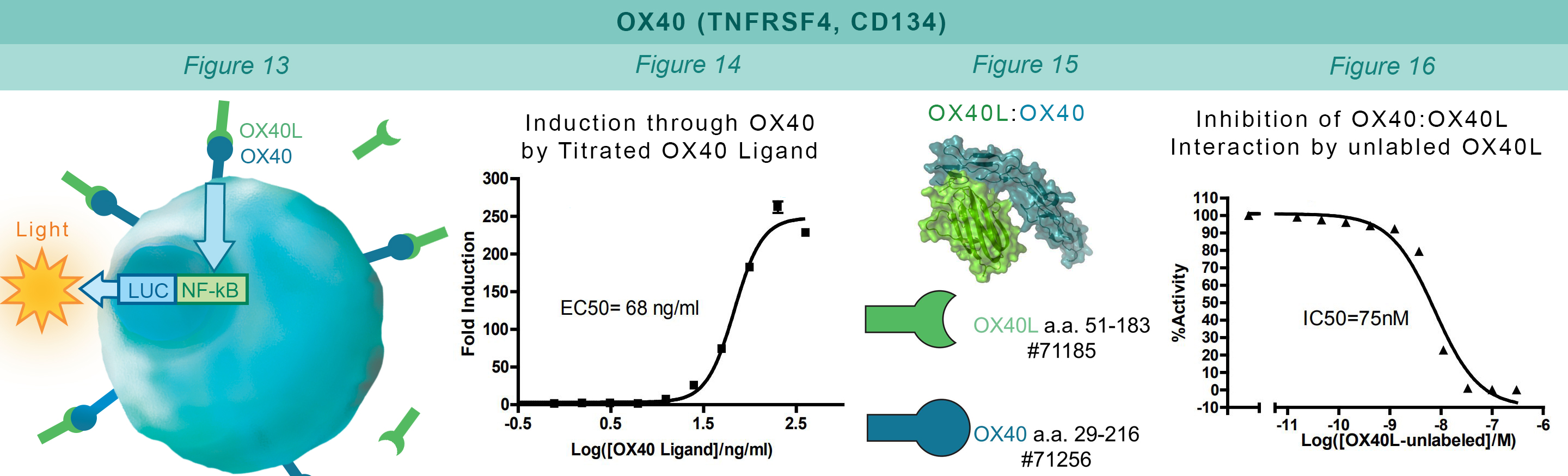
Similarly to the GITR assay, HEK293 cells constitutively expressing OX40 and an engineered luciferase gene controlled by NF-κB response elements are treated with soluble OX40L protein (#71185). The OX40:OX40L interaction stimulates luciferase expression (Figure 13 & Figure 14). The chemiluminescent, direct assay is used to monitor the protein-protein interaction between the purified, extracellular domains of OX40 and OX40L (Figure 15). Unlabeled OX40L inhibits the OX40:OX40L(Labeled) interaction (Figure 16).
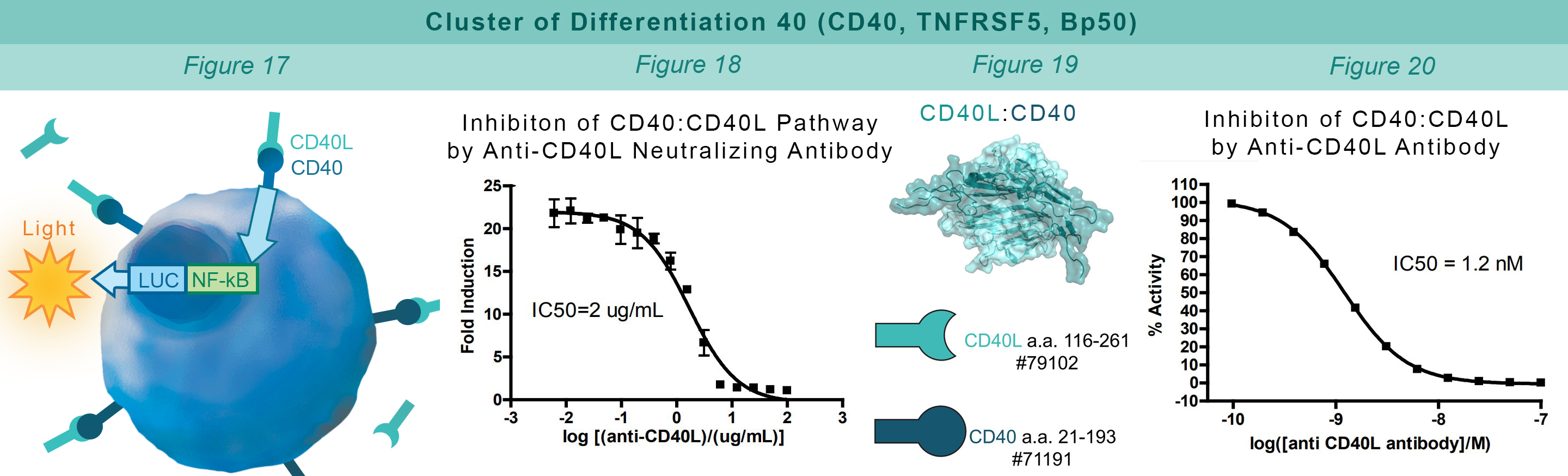
CD40 is found on B lymphocytes and APCs. Additionally, a variety of carcinoma cells over-express CD40. Interaction with CD40 ligand (CD40L, CD154) on CD4+ T helper lymphocytes triggers the expression of pro-inflammatory cytokines. HEK293 cells that constitutively express CD40 and an engineered luciferase gene controlled by NF-κB response elements are treated with soluble CD40L protein (#71191). The CD40:CD40L interaction stimulates luciferase expression (Figure 17). Anti-CD40 antibody inhibits this CD40:CD40L interaction. This prevents luciferase expression and production of reporter signal (Figure 18). The chemiluminescent, direct assay is used to monitor the protein-protein interaction between the purified, extracellular domains of CD40 and CD40L (Figure 19). The anti-CD40L antibody inhibits the CD40:CD40L interaction (Figure 20).
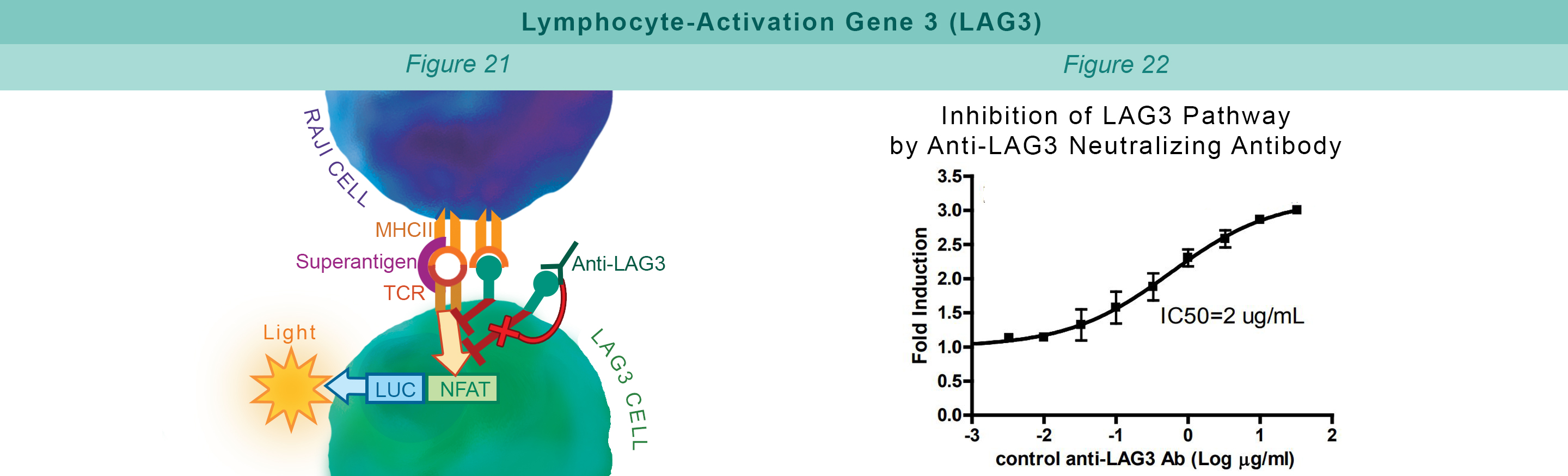
LAG3 is expressed on activated T cells, natural killer cells, B cells, and APCs. Its interaction with MHC class II negatively regulates cellular proliferation, activation, and homeostasis of T cells, and plays a role in Treg suppressive function. Jurkat cells constitutively expressing LAG3 and expressing firefly luciferase under the control of inducible NFAT response elements are co-cultured with RAJI cells, and incubated with Superantigen (Figure 21). The LAG3:MHCII interaction inhibits luciferase expression through TCR signaling. Anti-LAG3 antibody inhibits LAG3:MHCII interaction. This allows luciferase expression and production of reporter signal (Figure 22).
[1] Keir, M.E., et al. 2008, Annu. Rev. Immunol. 26: 677
[2]Zak, K.M., et al. 2015, Structure 23: 2341.
[3] Zak, K.M., et al. 2016, Oncotarget 7: 30323
[4] Yu, X., et al., 2009, Nat. Immunol. 10: 48
[5] Stengel, K. F., et al. 2012, Proc.Natl.Acad.Sci.USA 109: 5399
[6] Lechner, M.G., et al. 2011, Immunotherapy 3:1317
[7] Peng, K., et al. 2014, AAPS J. 16(4): 625
[8]Compaan, D.M. et al. 2006, Structure 14: 1321
[9] Li. G., et al. 2013, PLoS Genet. 9: e1003487
[10] An, H.J., et al. 2011, J.Biol.Chem. 286: 11226
[11] Byrnes, et al., 2012, Cytometry A. Sep;81:165
[12] Lob, S. et al., 2009, Cancer Immunol. Immunother. 58: 153
PD-1 Related Products
- PD-1:PD-L1/PD-L2 Cell-Based Inhibitor Screening Assay Kit (#60800)
- PD-1 / NFAT Reporter - Jurkat Recombinant Cell Line (#60535)
- PD-L1 / TCR Activator - CHO Recombinant Cell Line (#60536)
- Anti-PD-L1 (CD274) Neutralizing Antibody (#71120)
- PD-1:PD-L1 TR-FRET Assay - 384 rxns(#72038)
- PD-1:PD-L1 TR-FRET Assay - 96 rxns (#72032)
- PD-1:PD-L2 TR-FRET Assay Kit (#72012)
TIGIT Related
GITR Related Products
OX40 Related Products
CD40 Related Products
LAG3 Related Products
Technical Backgrounds