CD39 & CD73: Adenosine Signaling
Therapeutic antibodies targeting checkpoint receptors such as PD-1 and CTLA4 are giving new hope to cancer patients. However, not all patients respond to treatment and the search for effective co-therapies is driving substantial research. Two critical enzymes of this pathway, CD39 and CD73, are promising targets for increasing the effectiveness of checkpoint-based immunotherapies.1
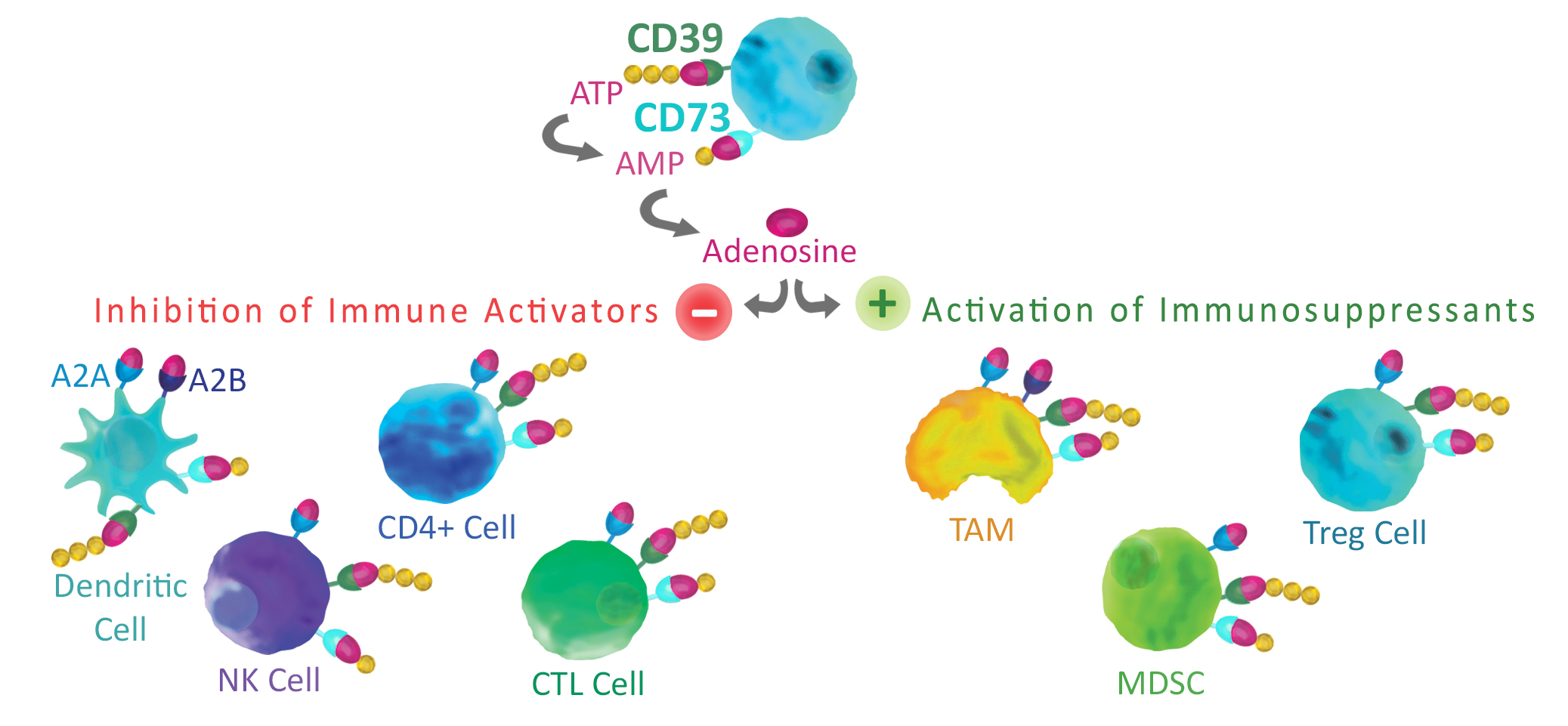
The hydrolysis of extracellular ATP to AMP is a well-known suppressor of the immune system.CD39 contributes to immune regulation through the hydrolysis of ATP to AMP in the rate limiting step of extracellular adenosine production2. CD39 is expressed on the surface of multiple cells of the immune system including T-cells, Natural killer cells, B-cells, dendritic cells and macrophages1. In addition, high levels of CD39 expression have been associated with the tumor microenvironment (TME). Research aimed at reducing immunosuppression by CD39 activity has resulted in the identification of a small molecule inhibitor, POM13, and several anti-CD39 antibodies selected that can block enzymatic activity4 or decrease CD39 expression5.
CD73 catalyzes the final hydrolysis of AMP to adenosine following CD39 activity2. Like CD39 it can be found on a wide range of immune cells including T-cells, B-cells, NK-cells and myeloid-derived suppressor cells1. Increased expression of CD73 has been associated with several tumor signaling pathways stimulated by hypoxyia in the TME6.
In mouse models, inhibition of CD73 with monoclonal antibodies is synergistic with inhibition of adenosine receptor signaling and shows promise as a co-therapy with anti-PD-1 and anti-CTLA4 treatments7. A crystal structure is also available of the active enzyme which can aid the search for small molecule inhibitors8 (see below and PDB:4H1S).
Once generated through the activity of CD39 and CD73, adenosine binds to A2A and A2B receptors expressed on tumor cells. This signaling can enhance tumor growth and directly promotes tumor cell proliferation. Additionally, adenosine inhibits anti-tumor cell activity through the inhibition of CD4+ cells, T cells, CTLs, dendritic cells, and NK cells. Adenosine also activates immunosuppressive cells such as Tregs, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs), allowing additional suppression of anti-tumor activity.
Development of inhibitory antibodies and small molecules against CD39 and CD73 are promising strategies in the treatment of cancer. This therapeutic potential is increased by the possibility of synergy with other targets such as PD-1, CTLA4, A2A and A2B. BPS Bioscience provides active, species-specific CD39 and CD73 proteins for both Human and Mouse models. Through use of the CD39 (#79278) and CD73 Inhibitor Screening Assay Kits (#72058 & #72055), activity can be studied in screening and profiling applications. In addition to these products, BPS also offers both CD39 and CD73 screening and profiling services to screen for neutralizing antibodies, small molecule inhibitors, inhibitors, determine IC50 values, and perform follow-up studies.
Whatever your project may entail, BPS Bioscience is here to provide the tools and project guidance to help you fulfill your research goals. Contact us today to learn more.
References:
[1] Allard, B., Longhi, M. S., Robson, S. C., and Stagg, J. (2017) The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets, Immunol Rev 276, 121-144.[2]Yegutkin, G. G. (2008) Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade, Biochim Biophys Acta 1783, 673-694.
[3] Muller, C. E., Iqbal, J., Baqi, Y., Zimmermann, H., Rollich, A., and Stephan, H. (2006) Polyoxometalates--a new class of potent ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) inhibitors, Bioorg Med Chem Lett 16, 5943-5947.
[4] Bastid, J., Cottalorda-Regairaz, A., Alberici, G., Bonnefoy, N., Eliaou, J. F., and Bensussan, A. (2013) ENTPD1/CD39 is a promising therapeutic target in oncology, Oncogene 32, 1743-1751.
[5] Nikolova, M., Carriere, M., Jenabian, M. A., Limou, S., Younas, M., Kok, A., Hue, S., Seddiki, N., Hulin, A., Delaneau, O., Schuitemaker, H., Herbeck, J. T., Mullins, J. I., Muhtarova, M., Bensussan, A., Zagury, J. F., Lelievre, J. D., and Levy, Y. (2011) CD39/adenosine pathway is involved in AIDS progression, PLoS Pathog 7, e1002110.
[6] Hatfield, S. M., Kjaergaard, J., Lukashev, D., Belikoff, B., Schreiber, T. H., Sethumadhavan, S., Abbott, R., Philbrook, P., Thayer, M., Shujia, D., Rodig, S., Kutok, J. L., Ren, J., Ohta, A., Podack, E. R., Karger, B., Jackson, E. K., and Sitkovsky, M. (2014) Systemic oxygenation weakens the hypoxia and hypoxia inducible factor 1alpha-dependent and extracellular adenosine-mediated tumor protection, J Mol Med (Berl) 92, 1283-1292.
[7] Young, A., Ngiow, S. F., Barkauskas, D. S., Sult, E., Hay, C., Blake, S. J., Huang, Q., Liu, J., Takeda, K., Teng, M. W., Sachsenmeier, K., and Smyth, M. J. (2016) Co-inhibition of CD73 and A2AR Adenosine Signaling Improves Anti-tumor Immune Responses, Cancer Cell 30, 391-403.
[8] Heuts, D. P., Weissenborn, M. J., Olkhov, R. V., Shaw, A. M., Gummadova, J., Levy, C., and Scrutton, N. S. (2012) Crystal structure of a soluble form of human CD73 with ecto-5'-nucleotidase activity, Chembiochem 13, 2384-2391.
Connect With BPS
Assay Data
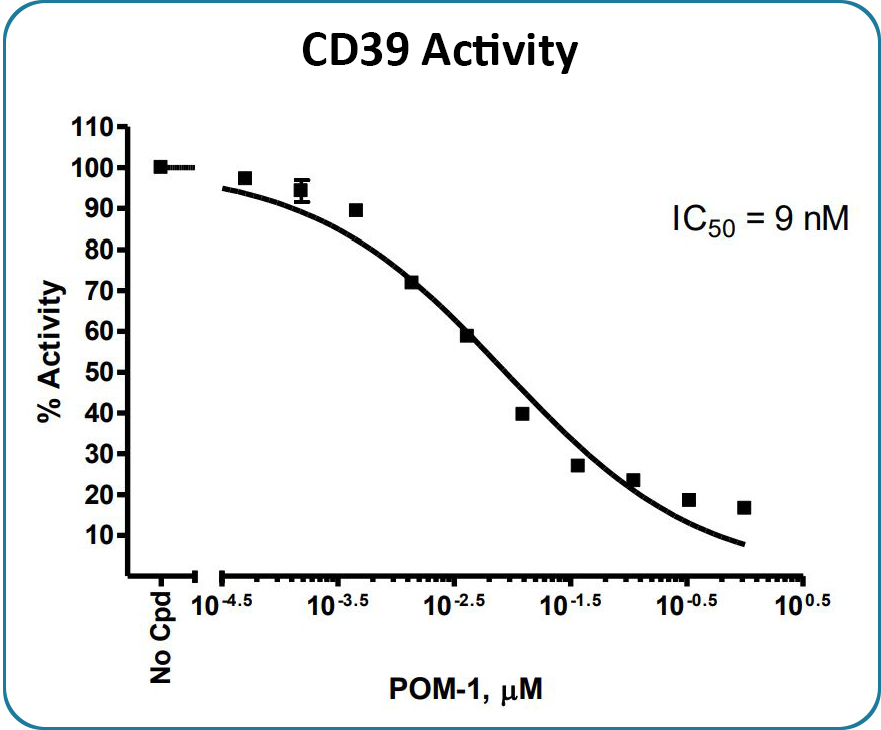
CD39 enzyme activity, measured using the CD39 Inhibitor Screening Assay Kit, Cat #79278