NAMPT: Metabolism, Cancer, and Drug Discovery
NAMPT & Metabolism
 The unique metabolic requirements of rapidly reproducing cells are classical targets for cancer treatment. For example, rapid growth creates a high demand for ATP used in the biosynthesis of DNA and proteins. The enzymatic cofactors NAD+ and NADH contribute oxidizing and reducing agents required to generate ATP and regulate the resulting oxidative stress. This need for high levels of ATP production makes the enzymatic pathways of sustaining NAD+ levels important to anti-cancer drug discovery.
The unique metabolic requirements of rapidly reproducing cells are classical targets for cancer treatment. For example, rapid growth creates a high demand for ATP used in the biosynthesis of DNA and proteins. The enzymatic cofactors NAD+ and NADH contribute oxidizing and reducing agents required to generate ATP and regulate the resulting oxidative stress. This need for high levels of ATP production makes the enzymatic pathways of sustaining NAD+ levels important to anti-cancer drug discovery.
Cells maintain adequate NAD+ levels through multiple enzymatic pathways. Nicotinamide phosphoribosyl transferase 1 (NAMPT) is the rate limiting enzyme of the primary pathway for maintaining cellular NAD+. The control function of this enzyme has made it an important target for drug discovery efforts designed to interfere with cancer metabolism 2.
NAMPT & Cancer
 A link between the enzymatic activity NAMPT and cancer metabolism is clearly established. NAMPT is overexpressed relative to non-cancerous cells in lymphomas 3 astrocytomas 4 and carcinomas 5. Furthermore, inhibitors of NAMPT activities have shown anti-tumor properties in several models of organ-specific cancers 6, 7.
A link between the enzymatic activity NAMPT and cancer metabolism is clearly established. NAMPT is overexpressed relative to non-cancerous cells in lymphomas 3 astrocytomas 4 and carcinomas 5. Furthermore, inhibitors of NAMPT activities have shown anti-tumor properties in several models of organ-specific cancers 6, 7.
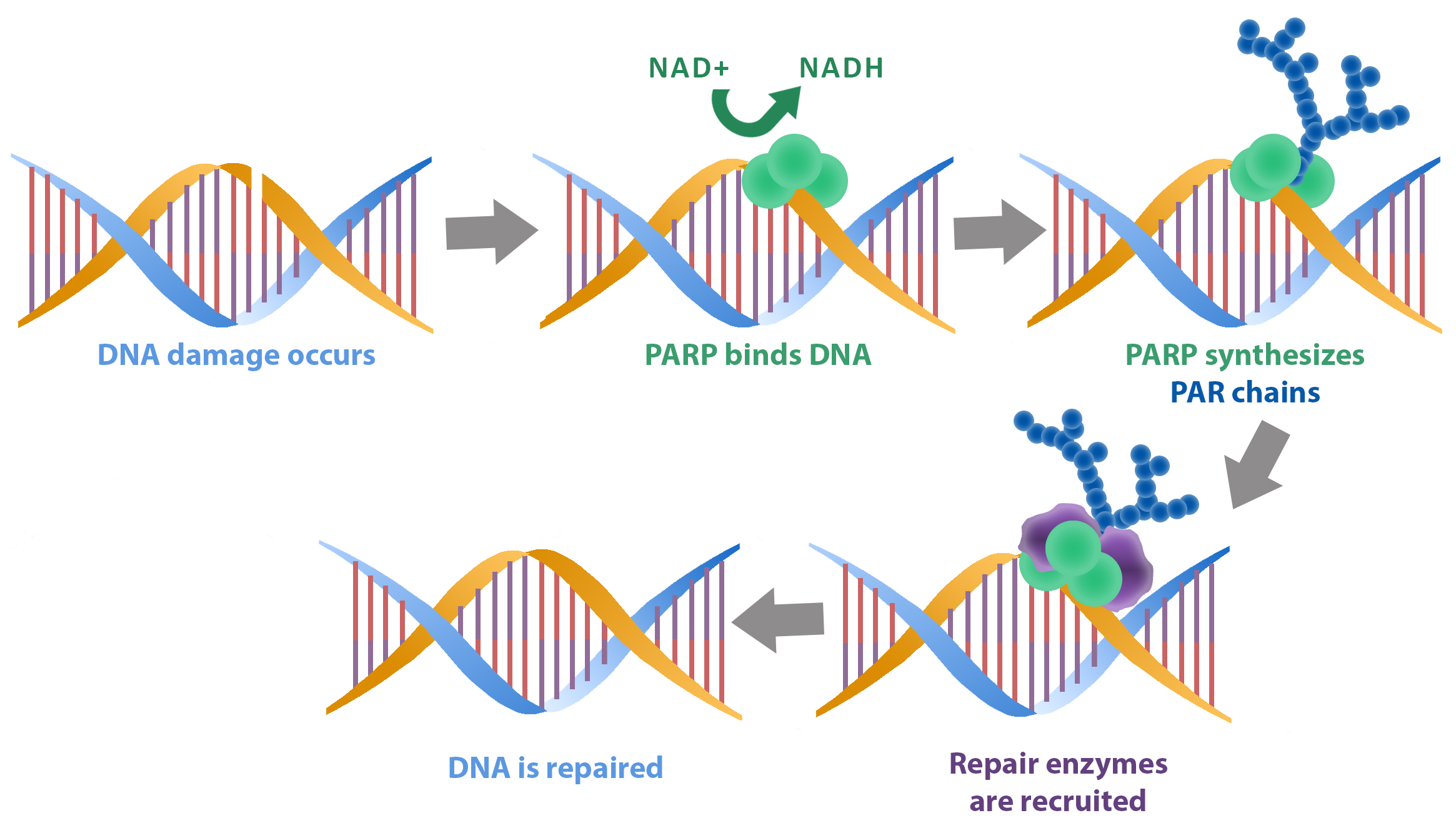
The control of NAD+ metabolism by NAMPT connects this enzyme with several other well established, anti-cancer targets that depend on NAD+ consumption for enzymatic function. PARPs are a family of 17 proteins that catalyze the NAD+ -dependent addition of poly(ADP-ribose) to proteins and are associated with response to DNA damage 8. Sirtuins include seven NAD+ dependent deacetylases associated with post-translational modification of tumor suppressors and transcription factors 9. Inhibition of NAMPT with small molecules contributes to both PARP 10, 11 and Sirtuin 12, 13 inhibition through NAD+ depletion. This suggests synergy between inhibitors of NAMPT and these classes of enzymes could lead to the development of potent co-inhibitors.
Because of the promise of NAMPT as an anti-cancer target, it has been the subject of intensive efforts to find inhibitors as drug candidates. Early inhibitors such as APO866/FK866 and GMX1778 have been extensively reported upon in the literature and studied in the clinic. These compounds demonstrate low nM inhibition of NAMPT 14-16. However, their toxicity profiles ultimately prevented them from reaching the therapeutic stage 2.
Drug Discovery
 Further advancements in the medicinal chemistry and dosing methods of NAMPT inhibitors continue to make this enzyme a highly relevant cancer target. The compound LSN3154567 is a potential breakthrough developed by a combination of screening and rational design 17. The result is an orally available compound that, when co-administered with the NAD+ precursor nicotinic acid, does not show the retinal and heamatological toxicities observed with older NAMPT inhibitors 17.
Further advancements in the medicinal chemistry and dosing methods of NAMPT inhibitors continue to make this enzyme a highly relevant cancer target. The compound LSN3154567 is a potential breakthrough developed by a combination of screening and rational design 17. The result is an orally available compound that, when co-administered with the NAD+ precursor nicotinic acid, does not show the retinal and heamatological toxicities observed with older NAMPT inhibitors 17.
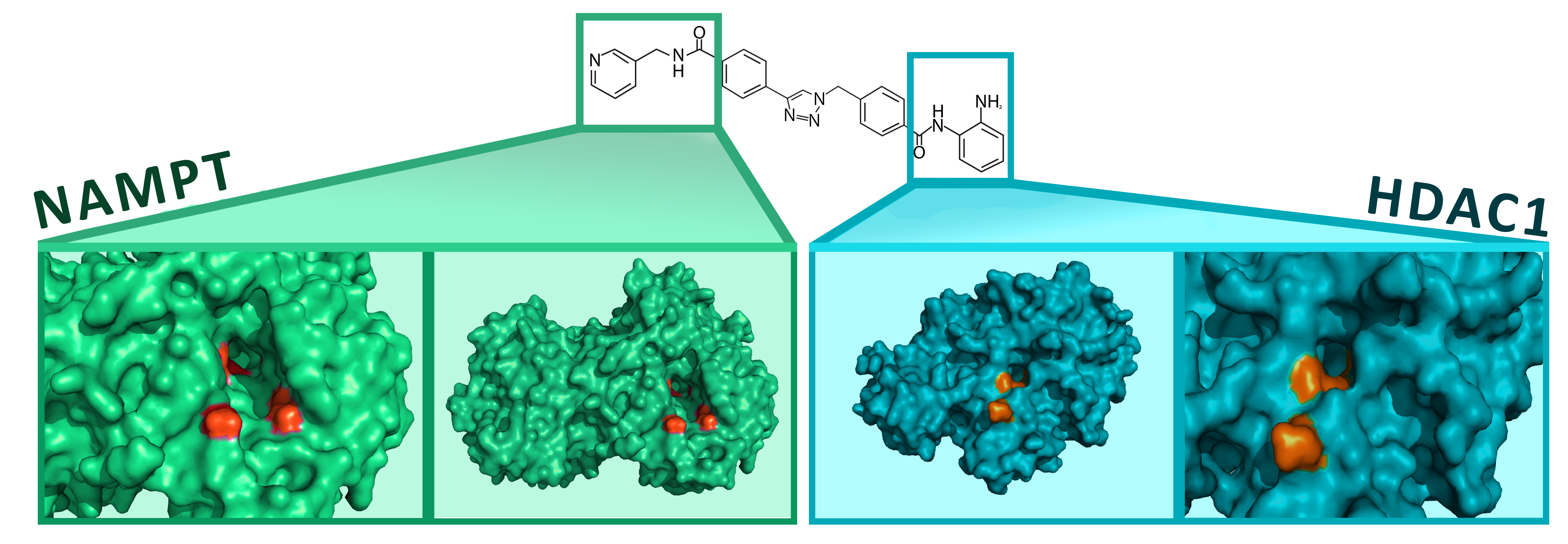
Another cleaver approach yielded the development of a dual NAMPT, Histone Deacetylase -1 (HDAC1) inhibitor 18. Functional groups from known NAMPT and HDAC1 inhibitors where combined through a chemical linker (See figure below). This molecule was then optimized through rounds of medicinal chemistry and inhibition assays. The resulting molecule is a dual inhibitor with nM potencies against both enzymes. While still a proof of concept, this novel compound has the potential to capitalize on the synergistic inhibition of two well understood, anti-cancer targets.
 References
References
[1] Revollo, J. R., Grimm, A. A., and Imai, S. (2004) The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells, J Biol Chem 279, 50754-50763.
[2] Roulston, A., and Shore, G. C. (2016) New strategies to maximize therapeutic opportunities for NAMPT inhibitors in oncology, Mol Cell Oncol 3, e1052180.
[3] Olesen, U. H., Hastrup, N., and Sehested, M. (2011) Expression patterns of nicotinamide phosphoribosyltransferase and nicotinic acid phosphoribosyltransferase in human malignant lymphomas, APMIS 119, 296-303.
[4] Reddy, P. S., Umesh, S., Thota, B., Tandon, A., Pandey, P., Hegde, A. S., Balasubramaniam, A., Chandramouli, B. A., Santosh, V., Rao, M. R., Kondaiah, P., and Somasundaram, K. (2008) PBEF1/NAmPRTase/Visfatin: a potential malignant astrocytoma/glioblastoma serum marker with prognostic value, Cancer biology & therapy 7, 663-668.
[5] Tian, W., Zhu, Y., Wang, Y., Teng, F., Zhang, H., Liu, G., Ma, X., Sun, D., Rohan, T., and Xue, F. (2013) Visfatin, a potential biomarker and prognostic factor for endometrial cancer, Gynecol Oncol 129, 505-512.
[6] Shackelford, R. E., Bui, M. M., Coppola, D., and Hakam, A. (2010) Over-expression of nicotinamide phosphoribosyltransferase in ovarian cancers, Int J Clin Exp Pathol 3, 522-527.
[7] Van Beijnum, J. R., Moerkerk, P. T., Gerbers, A. J., De Bruine, A. P., Arends, J. W., Hoogenboom, H. R., and Hufton, S. E. (2002) Target validation for genomics using peptide-specific phage antibodies: a study of five gene products overexpressed in colorectal cancer, Int J Cancer 101, 118-127.
[8] Gupte, R., Liu, Z., and Kraus, W. L. (2017) PARPs and ADP-ribosylation: recent advances linking molecular functions to biological outcomes, Genes Dev 31, 101-126.
[9] Jiang, Y., Liu, J., Chen, D., Yan, L., and Zheng, W. (2017) Sirtuin Inhibition: Strategies, Inhibitors, and Therapeutic Potential, Trends Pharmacol Sci 38, 459-472.
[10] Chan, M., Gravel, M., Bramoulle, A., Bridon, G., Avizonis, D., Shore, G. C., and Roulston, A. (2014) Synergy between the NAMPT inhibitor GMX1777(8) and pemetrexed in non-small cell lung cancer cells is mediated by PARP activation and enhanced NAD consumption, Cancer Res 74, 5948-5954.
[11] Kato, H., Ito, E., Shi, W., Alajez, N. M., Yue, S., Lee, C., Chan, N., Bhogal, N., Coackley, C. L., Vines, D., Green, D., Waldron, J., Gullane, P., Bristow, R., and Liu, F. F. (2010) Efficacy of combining GMX1777 with radiation therapy for human head and neck carcinoma, Clin Cancer Res 16, 898-911.
[12] Venkateshaiah, S. U., Khan, S., Ling, W., Bam, R., Li, X., van Rhee, F., Usmani, S., Barlogie, B., Epstein, J., and Yaccoby, S. (2013) NAMPT/PBEF1 enzymatic activity is indispensable for myeloma cell growth and osteoclast activity, Exp Hematol 41, 547-557 e542.
[13] Wang, B., Hasan, M. K., Alvarado, E., Yuan, H., Wu, H., and Chen, W. Y. (2011) NAMPT overexpression in prostate cancer and its contribution to tumor cell survival and stress response, Oncogene 30, 907-921.
[14] Binderup, E., Bjorkling, F., Hjarnaa, P. V., Latini, S., Baltzer, B., Carlsen, M., and Binderup, L. (2005) EB1627: a soluble prodrug of the potent anticancer cyanoguanidine CHS828, Bioorg Med Chem Lett 15, 2491-2494.
[15] Hasmann, M., and Schemainda, I. (2003) FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis, Cancer Res 63, 7436-7442.
[16] Olesen, U. H., Christensen, M. K., Bjorkling, F., Jaattela, M., Jensen, P. B., Sehested, M., and Nielsen, S. J. (2008) Anticancer agent CHS-828 inhibits cellular synthesis of NAD, Biochem Biophys Res Commun 367, 799-804.
[17] Zhao, G., Green, C. F., Hui, Y. H., Prieto, L., Shepard, R., Dong, S., Wang, T., Tan, B., Gong, X., Kays, L., Johnson, R. L., Wu, W., Bhattachar, S., Del Prado, M., Gillig, J. R., Fernandez, M. C., Roth, K. D., Buchanan, S., Kuo, M. S., Geeganage, S., and Burkholder, T. P. (2017) Discovery of a Highly Selective NAMPT Inhibitor That Demonstrates Robust Efficacy and Improved Retinal Toxicity with Nicotinic Acid Co-administration, Mol Cancer Ther.
[18] Dong, G., Chen, W., Wang, X., Yang, X., Xu, T., Wang, P., Zhang, W., Rao, Y., Miao, C., and Sheng, C. (2017) Small Molecule Inhibitors Simultaneously Targeting Cancer Metabolism and Epigenetics: Discovery of Novel Nicotinamide Phosphoribosyltransferase (NAMPT) and Histone Deacetylase (HDAC) Dual Inhibitors, J Med Chem 60, 7965-7983.
[19] Khan, J. A., Tao, X., and Tong, L. (2006) Molecular basis for the inhibition of human NMPRTase, a novel target for anticancer agents, Nat Struct Mol Biol 13, 582-588.
[20] Millard, C. J., Watson, P. J., Celardo, I., Gordiyenko, Y., Cowley, S. M., Robinson, C. V., Fairall, L., and Schwabe, J. W. (2013) Class I HDACs share a common mechanism of regulation by inositol phosphates, Mol Cell 51, 57-67
Assay Data
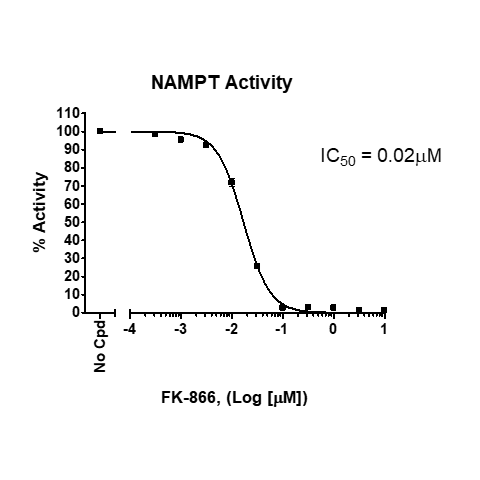
NAMPT inhibition by FK866, measured using the NAMPT Inhibitor Screening Assay Kit, BPS Bioscience Cat. #71276.
Proteins
Assay Kits
Services