TIGIT, CD155, & CD226
Promising Targets for Cancer Immunotherapies
It’s possible someone you know has benefited from cancer treatments based on checkpoint receptors. Therapeutic antibodies targeting PD-1 and CTLA-4 pathways have already added years to the lives of lucky patients. Unfortunately, not everyone responds to these treatments. This resistance drives the search for new therapies to extend the successes of existing PD-1 and CTLA-4-based treatments.
NK and T cells are partially regulated by the checkpoint receptors CD155, CD226, and TIGIT1-3. This system closely resembles immune regulation through the CD28/CD80/CTLA-4 system4. In the simplest terms, interactions between CD155 and CD226 stimulate the immune system through positive regulation of NK and T cells. TIGIT competes with CD226 for binding with CD155, and this results in immune suppression based on both competitive interaction and intracellular signaling through TIGIT.
CD155 and TIGIT in Cancer
CD155 is highly expressed on tumor cells, and has increased expression in several cancers including lung, breast, ovarian, and melanoma5-8. As a biomarker, a high level of CD155 expression correlates with poor prognosis in cancer patients9. TIGIT expression is upregulated on tumor infiltrating lymphocytes (TILs) and is highly expressed on TILs associated with multiple cancers10-12. The contributions of CD155 and TIGIT to immune evasion makes them promising targets for development of new therapeutic antibodies.
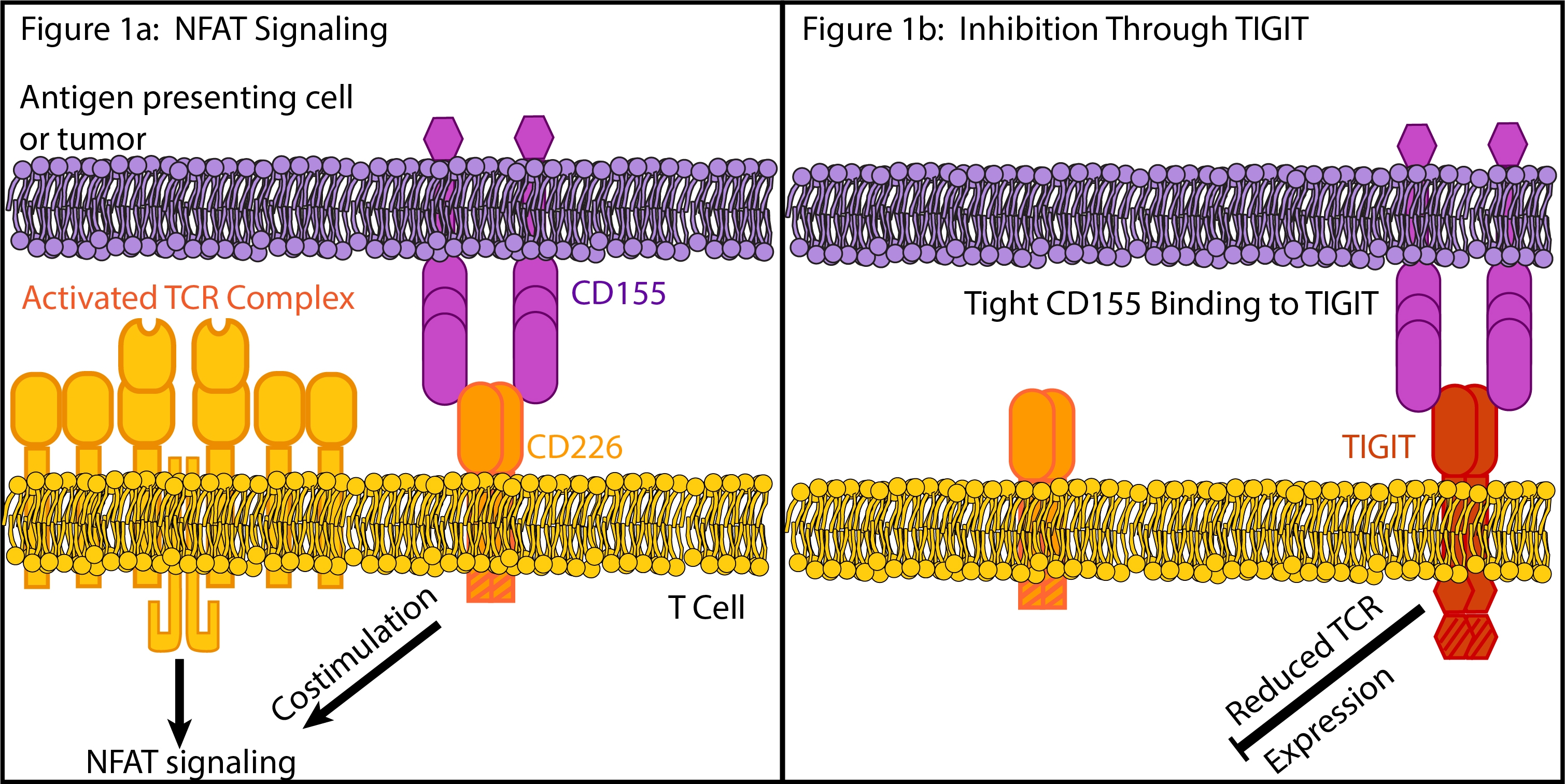
Domain Structure and Function of CD155, CD226, and TIGIT
When targeting the CD155, CD226, and TIGIT system, it is helpful to understand the basics of their structure and function. Early studies of CD155 focused on its contribution to polio virus infection13. It wasn’t until decades later that its biological functions and roles in cancer were better understood. CD155 is a lectin-like protein with three extracellular immunoglobulin (Ig) domains, a transmembrane domain, and an intracellular domain14. Epithelial, endothelial, and immune cells express CD155 at low levels3. Its expression increases in response to LPS and inflammatory cytokines consistent with function as part of the innate immune system3.
CD226 is an immune stimulator that regulates NK and T cell function15. T Cell receptor (TCR) engagement leads to productive T cell activation through the NFAT pathway when coupled with costimulatory signals such as those delivered through CD226 when bound by CD155 (Fig1a). CD226 is a member of the immunoglobulin (Ig) superfamily with two extra cellular Ig-like domains and multiple glycosylation sites15. It has a singular transmembrane domain and an intracellular domain with multiple phosphorylation sites. CD226 does not contain a traditional immunoreceptor tyrosine-based activator domain, and its exact signaling mechanisms are unknown. However, they do require phosphorylation of CD226 by protein kinase C16.
TIGIT is an immune system inhibitor expressed on NK cells and a variety of T cells including CD4+ T cells, Treg, and CD8+ T cells11, 14. Similar to CD226, TIGIT is a member of the immunoglobulin superfamily with an extracellular Ig domain, a single transmembrane domain, and intracellular immunoreceptor tyrosine-based inhibitor (ITIM) and Ig tail-tyrosine (ITT)-like motifs14, 17.
Mechanism and Structure in Immune Regulation by the CD155 and TIGIT Complex
 The mechanistic and structural details of the CD155 interaction with TIGIT are critical for understanding immune inhibition through this system. In T cells, multiple mechanisms are associated with immunosuppression. CD155 binds to TIGIT with a higher affinity than CD226 14. This competitive interaction serves to inhibit stimulation through CD226 by sequestering CD155 in complexes with TIGIT (Fig 1b). Possibly more importantly, TIGIT engagement also leads to downregulated expression of the components of the TCR complex including the a-chain and CD311 Fig(1b). Thus TIGIT blocks NFAT signaling pathway potentially by both preventing CD226 engagement and by directly targeting formation of the TCR complex.
The mechanistic and structural details of the CD155 interaction with TIGIT are critical for understanding immune inhibition through this system. In T cells, multiple mechanisms are associated with immunosuppression. CD155 binds to TIGIT with a higher affinity than CD226 14. This competitive interaction serves to inhibit stimulation through CD226 by sequestering CD155 in complexes with TIGIT (Fig 1b). Possibly more importantly, TIGIT engagement also leads to downregulated expression of the components of the TCR complex including the a-chain and CD311 Fig(1b). Thus TIGIT blocks NFAT signaling pathway potentially by both preventing CD226 engagement and by directly targeting formation of the TCR complex.
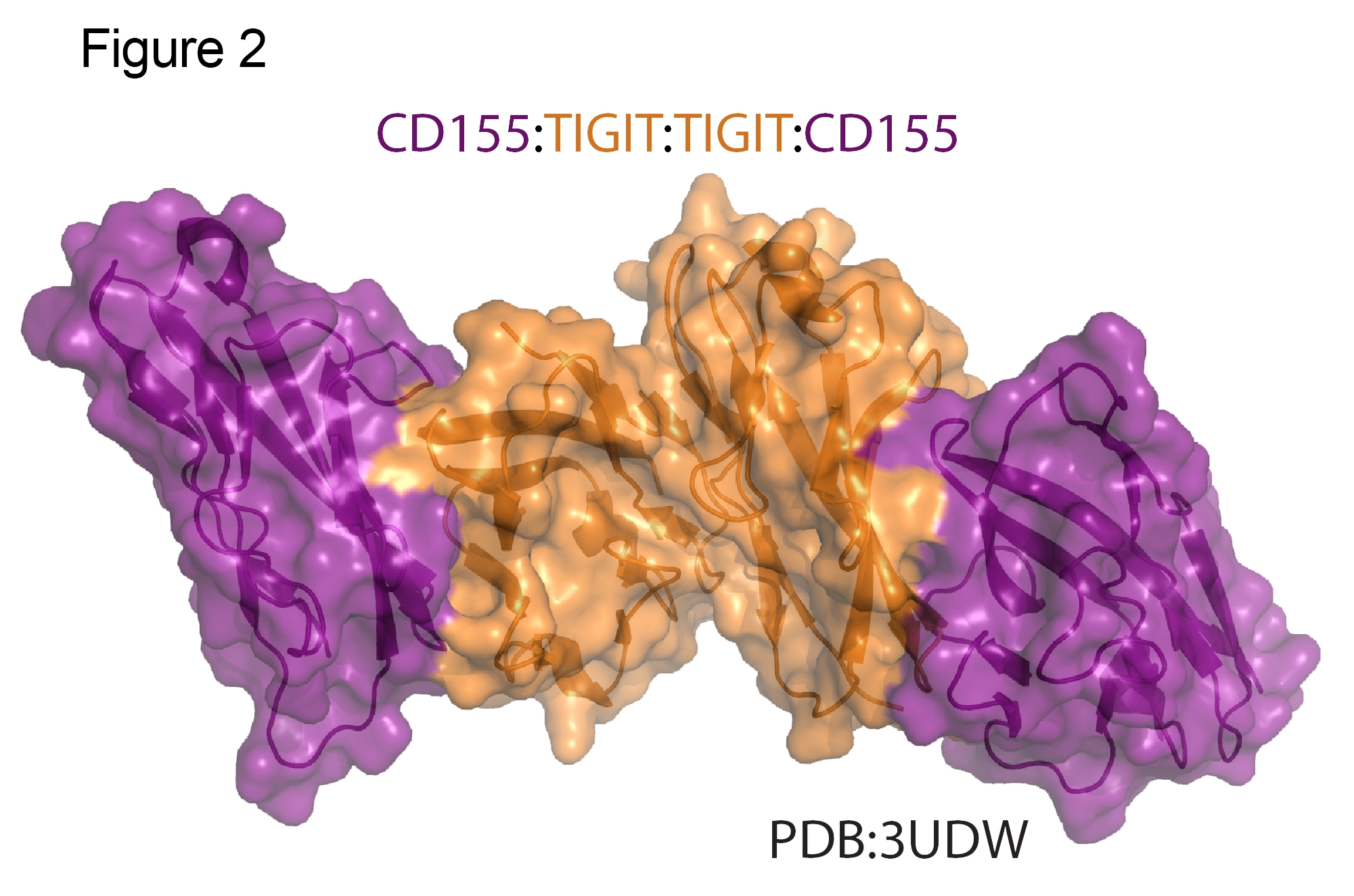
The extra cellular Ig domains of CD155 and TIGIT form a heterotetramer containing two molecules of both proteins18. In this complex, TIGIT forms a homodimer flanked by CD155 monomers (Fig 2). Inhibitory signaling requires homodimerization of TIGIT18. The distinct interface between the TIGIT dimer interface and the CD155 binding site are independent, potential targets for disruption with therapeutic antibodies. The atomic level understanding of this complex provides valuable information for the structure based design of both antibody and biologic therapeutics.
Understanding and targeting CD155, CD226 and TIGIT interactions becomes a bit more complicated when considering the additional receptors that join the party. CD112 is an immune stimulator that functions like CD155 through binding with both CD226 and TIGIT3. Another protein, CD96, acts like TIGIT as an immune suppressor through interactions with both CD155, CD112, and CD112 homologues called CD111 and CD1133. This promiscuity increases the opportunities for developing immunotherapies; however, it also provides greater risk of complications from unintended cross reactivity. Because of this complexity, high quality research tools are essential to reach the full therapeutic potential of the CD155, CD226, and TIGIT system.
References
[1] Bowers, J. R., Readler, J. M., Sharma, P., and Excoffon, K. (2017) Poliovirus Receptor: More than a simple viral receptor, Virus Res 242, 1-6.
[2] de Andrade, L. F., Smyth, M. J., and Martinet, L. (2014) DNAM-1 control of natural killer cells functions through nectin and nectin-like proteins, Immunol Cell Biol 92, 237-244.
[3] Dougall, W. C., Kurtulus, S., Smyth, M. J., and Anderson, A. C. (2017) TIGIT and CD96: new checkpoint receptor targets for cancer immunotherapy, Immunol Rev 276, 112-120.
[4] Ribas, A., and Wolchok, J. D. (2018) Cancer immunotherapy using checkpoint blockade, Science 359, 1350-1355.
[5] Casado, J. G., Pawelec, G., Morgado, S., Sanchez-Correa, B., Delgado, E., Gayoso, I., Duran, E., Solana, R., and Tarazona, R. (2009) Expression of adhesion molecules and ligands for activating and costimulatory receptors involved in cell-mediated cytotoxicity in a large panel of human melanoma cell lines, Cancer Immunol Immunother 58, 1517-1526.
[6] Gromeier, M., Lachmann, S., Rosenfeld, M. R., Gutin, P. H., and Wimmer, E. (2000) Intergeneric poliovirus recombinants for the treatment of malignant glioma, Proc Natl Acad Sci U S A 97, 6803-6808.
[7] Masson, D., Jarry, A., Baury, B., Blanchardie, P., Laboisse, C., Lustenberger, P., and Denis, M. G. (2001) Overexpression of the CD155 gene in human colorectal carcinoma, Gut 49, 236-240.
[8] Nakai, R., Maniwa, Y., Tanaka, Y., Nishio, W., Yoshimura, M., Okita, Y., Ohbayashi, C., Satoh, N., Ogita, H., Takai, Y., and Hayashi, Y. (2010) Overexpression of Necl-5 correlates with unfavorable prognosis in patients with lung adenocarcinoma, Cancer Sci 101, 1326-1330.
[9] Iguchi-Manaka, A., Okumura, G., Kojima, H., Cho, Y., Hirochika, R., Bando, H., Sato, T., Yoshikawa, H., Hara, H., Shibuya, A., and Shibuya, K. (2016) Increased Soluble CD155 in the Serum of Cancer Patients, PLoS One 11, e0152982.
[10] Johnston, R. J., Comps-Agrar, L., Hackney, J., Yu, X., Huseni, M., Yang, Y., Park, S., Javinal, V., Chiu, H., Irving, B., Eaton, D. L., and Grogan, J. L. (2014) The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function, Cancer Cell 26, 923-937.
[11] Joller, N., Hafler, J. P., Brynedal, B., Kassam, N., Spoerl, S., Levin, S. D., Sharpe, A. H., and Kuchroo, V. K. (2011) Cutting edge: TIGIT has T cell-intrinsic inhibitory functions, J Immunol 186, 1338-1342.
[12] Kurtulus, S., Sakuishi, K., Ngiow, S. F., Joller, N., Tan, D. J., Teng, M. W., Smyth, M. J., Kuchroo, V. K., and Anderson, A. C. (2015) TIGIT predominantly regulates the immune response via regulatory T cells, J Clin Invest 125, 4053-4062.
[13] Holland, J. J., and Mc, L. L. (1961) The location and nature of enterovirus receptors in susceptible cells, J Exp Med 114, 161-171.
[14] Yu, X., Harden, K., Gonzalez, L. C., Francesco, M., Chiang, E., Irving, B., Tom, I., Ivelja, S., Refino, C. J., Clark, H., Eaton, D., and Grogan, J. L. (2009) The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells, Nat Immunol 10, 48-57.
[15] Vivier, E., Tomasello, E., Baratin, M., Walzer, T., and Ugolini, S. (2008) Functions of natural killer cells, Nat Immunol 9, 503-510.
[16] Shibuya, A., Lanier, L. L., and Phillips, J. H. (1998) Protein kinase C is involved in the regulation of both signaling and adhesion mediated by DNAX accessory molecule-1 receptor, J Immunol 161, 1671-1676.
[17] Levin, S. D., Taft, D. W., Brandt, C. S., Bucher, C., Howard, E. D., Chadwick, E. M., Johnston, J., Hammond, A., Bontadelli, K., Ardourel, D., Hebb, L., Wolf, A., Bukowski, T. R., Rixon, M. W., Kuijper, J. L., Ostrander, C. D., West, J. W., Bilsborough, J., Fox, B., Gao, Z., Xu, W., Ramsdell, F., Blazar, B. R., and Lewis, K. E. (2011) Vstm3 is a member of the CD28 family and an important modulator of T-cell function, Eur J Immunol 41, 902-915.
[18] Stengel, K. F., Harden-Bowles, K., Yu, X., Rouge, L., Yin, J., Comps-Agrar, L., Wiesmann, C., Bazan, J. F., Eaton, D. L., and Grogan, J. L. (2012) Structure of TIGIT immunoreceptor bound to poliovirus receptor reveals a cell-cell adhesion and signaling mechanism that requires cis-trans receptor clustering, Proc Natl Acad Sci U S A 109, 5399-5404.








