PARP1 Knockout HeLa Cell Line
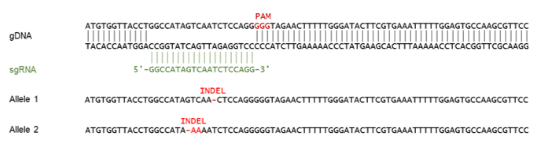
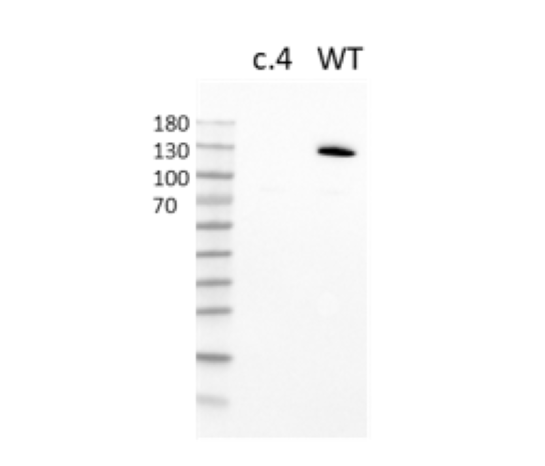
PARP1 Knockout HeLa Cell Line is a HeLa cell line in which PARP1 (Poly-(ADP-ribose) Polymerase 1) has been genetically removed with CRISPR/Cas9 genome editing.
This cell line has been validated by genomic sequencing and Western Blot analysis.
Purchase of this cell line is for research purposes only; commercial use requires a separate license. View the full terms and conditions.
Media Required for Cell Culture
| Name | Ordering Information |
| Thaw Medium 6 | BPS Bioscience #60183 |
The cell line has been screened to confirm the absence of Mycoplasma species.
PARP1, also known as poly-(ADP-ribose) polymerase 1 or NAD+ ADP-ribosyltransferase 1, is part of the PARP family, and it is the most abundant member. ADP ribosylation, which is the addition of an ADP-ribose to a protein, is a reversible post-translational modification of proteins mostly involved in the DNA Damage Response (DDR) pathway. Poly-ADP-ribosylation (termed PARylation) is the addition of linear or branched chains of ADP-ribose. PARP1 participates in DNA repair by non-homologous end joining (NHEJ), homologous recombination (HR), microhomology-mediated end-joining (MMEJ) and nucleotide excision repair. Dysfunction of DDR pathways can lead to oncogenesis. Overexpression of PARP1 has been found in breast and colon cancer, neuroblastoma, and others. This overexpression can lead to increasing MMEJ, an error-prone DNA repair mechanism, and genome instability leading to cancer. In addition to being involved in DDR, PARP1 is also linked to inflammation and type I diabetes. PARP1 inhibitors have been used in cancer treatment with success. In addition to reducing MMEJ, the use of PARP1 inhibitors can lead to synthetic lethality when homologous recombination repair (HRR) mechanisms are already defective, as in the case of BRCA1 (breast cancer susceptibility protein type 1) and BRCA2 deficient cells. Further understanding of the molecular pathways involving PARP1, and this contribution to disease, will continue to pave the way for new therapies for PARP1-linked diseases.
Marques M., et al., 2019 Oncogene 38 (12): 2177-2191.