Nicotinamide Adenine Dinucleotide
Overview
NAD+ plays an important role as a cofactor in enzyme catalyzing redox reactions by transferring electrons to/from its reduced form, NADH. It also serves as a substrate for various enzymes such as Sirtuins, Poly ADP-ribose polymerases (PARPs), and CD38.
Clinical Importance
Recent studies revealed that cellular NAD+ concentration is associated with chronological aging and age-related diseases, suggesting it is important to understand NAD+ metabolism and its regulation. (1) (2)
NAD+ Biosynthesis
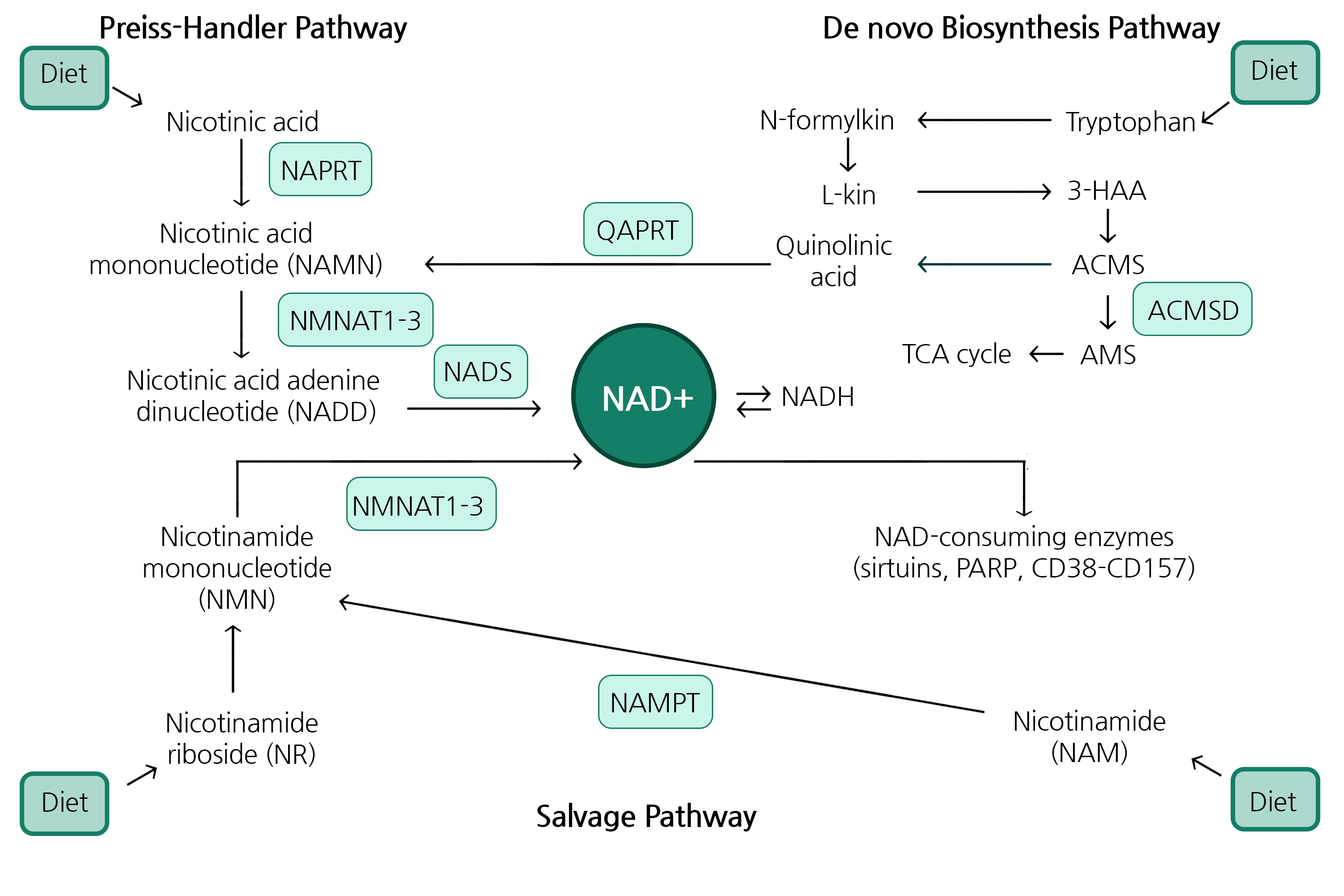
In cells, NAD+ is synthesized via de novo synthesis starting from tryptophan, the classical Preiss-Handler pathway, or the NAD+ salvage pathway that recycles nicotinamide produced as a byproduct by NAD+-consuming enzymes.
NAD+ Consuming Enzymes
Sirtuins are a group of enzymes that play an important role in aging and longevity by catalyzing NAD+-dependent deacylation/deacetylation of various substrate proteins. It has been suggested that decrease in the cellular NAD+ level by other NAD-consuming enzymes such as PARP1 and CD38 limits NAD+ availability for Sirtuins, causing metabolic disorders, cardiovascular and neurodegenerative diseases. More recently, another NAD+ consuming enzyme, SARM1, was shown to promote axonal degeneration via NAD+ hydrolysis.
NAD+ Producing Enzymes
A major pathway to synthesize NAD+ in mammalian cells is the salvage pathway from nicotinamide produced by NAD+-consuming enzymes. Nicotinamide is converted to nicotinamide mononucleo-tide (NMN) by nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme in this pathway. In various cancer cells as well as in active immune cells, NAMPT is highly expressed to maintain the NAD+ level, suggesting NAMPT may be a promising target for cancer and autoimmune disease treatments.
References