FAP Assay Service
●
Target
FAP
●
Description
Screening and/or profiling inhibitor compounds against FAP (fibroblast activation protein) protease activity in a biochemical assay.
●
Synonyms
Prolyl endopeptidase FAP, 170 kDa melanoma membrane-bound gelatinase, Dipeptidyl peptidase FAP, Fibroblast activation protein alpha, FAPalpha, Gelatine degradation protease FAP, Integral membrane serine protease, Post-proline cleaving enzyme
●
Example Data
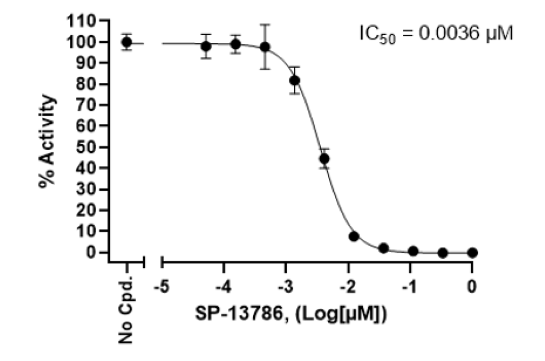
*Example only, final data may vary.
Assay Details
●
Assay Format
Fluorogenic
●
Reference Compounds and IC50
SP-13786, 3.6 nM
●
Assay Principle
The FAP Fluorogenic Assay is designed to measure FAP (fibroblast activation protein) protease activity for screening and profiling applications. A fluorogenic peptide substrate (Ala-Pro-AMC dipeptide) is used to measure FAP acitivity. In the conjugated form the energy emitted from the fluorochrome AMC is quenched. Proteolysis releases AMC and fluorescence is emitted. The increase in fluorescence is directly proportional to FAP activity, which is measured using a fluorescence reader.
Target Details
●
Protein Family
Proteases
●
UniProt
Q12884
●
Background
Fibroblast Activation Protein, or FAP, is a type II membrane serine protease of the dipeptidyl peptidase (DPP) subfamily. It is involved in wound healing and tissue repair. FAP upregulation is seen on activated stromal fibroblasts present in cancer, including breast, lung, prostate, pancreatic and cervical cancer. It has an impact on the tumor microenvironment (TME), where it reduces the levels of PEDF (pigment epithelium derived factor), angiopoietin-1 and VEGF-c (vascular endothelial growth factor c) and increase TGF-β (transforming growth factor β) and create an immunosuppressive environment. It is used as biomarker for pro-tumorigenic stroma and links to a poor prognosis. Studies in animal models have shown that knock-out of FAP reduces the metastasis of pancreatic ductal adenocarcinomas (PDA). It has thus been proposed as a candidate target for small molecule inhibitors for cancer therapy applications. Additionally, its expression profile makes it a good candidate for CAR-T applications, with pre-clinical and clinical trials currently ongoing.
Delivery
●
Estimated Turnaround
Two to three weeks following delivery of compounds
●
Results
Extensive report with raw and analyzed data, graphs, and detailed protocols. Includes positive control for inhibition.

