Combining Therapies for Targeting Immune Checkpoints
| IMMUNE CHECKPOINTS | | KINASES | | PARPS | | HDACS | | CAR T-CELL THERAPY |
Cancer is a balance between the defenses of the immune system and adaptations of diseased cells. In early stages, the immune system destroys most cancerous cells (1). Unfortunately, some cancers survive by hijacking the molecules that regulate immunity. This contributes to an immunosuppressive tumor microenvironment (TME) where tumors can grow without restriction. Decades of research have produced therapies designed to overcome the suppressive TME. Among the best of these are therapeutic antibodies that enhance T cell responses (2). These therapeutic antibodies can be life extending and provide genuine hope to patients with cancers with high mortality rates.
Still, cancer is relentless. Many patients are not responsive to therapeutic antibodies, and their cancers remain untreatable (3). Cancerous cells also evolve resistance under the selective pressure of treatment. Cancer is usually too complex and adaptable to be cured by monotherapies.
Much of the current research on therapeutic antibodies is aimed at finding combination treatments to overcome the limitations of monotherapies. More often than not, the adaptability of cancers makes them too difficult to treat by a single method or a one size fits all approach.
Similar to “drug cocktails” for treating HIV, improving combinations of cancer co-therapies make disease more manageable. BPS Bioscience aims to advance this research by providing tools such as recombinant proteins, biochemical and cellular assays, and specialized cell lines that speed the discovery of next generation immunotherapies.
The most important therapeutic antibodies target immunomodulating receptors expressed on cellular surfaces that regulate the interaction between the cells of the immune system and the rest of the body. The two most effective targets are the checkpoint inhibitors CTLA4 and PD-1.
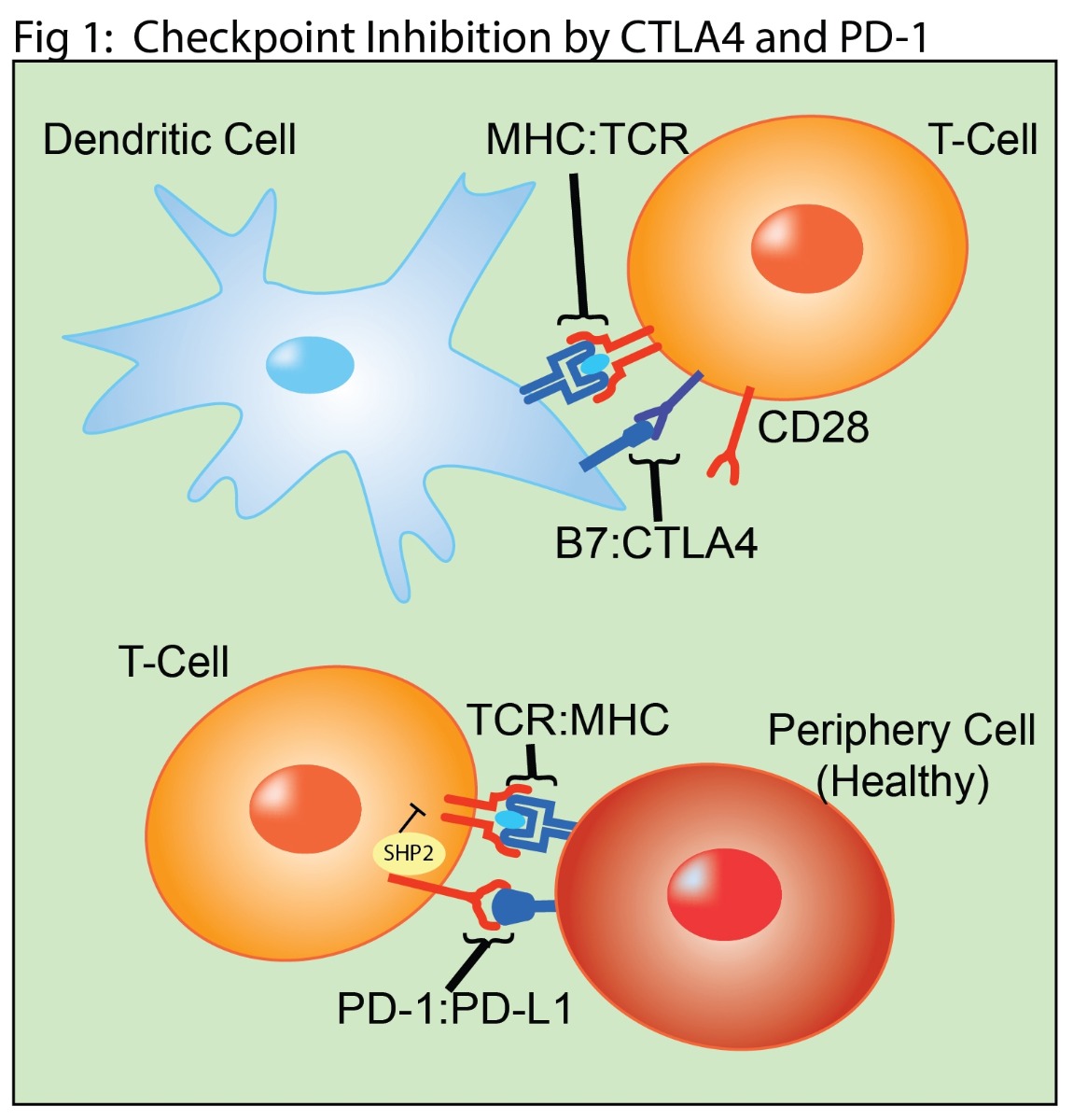
In normal function, T cells are activated by co-signaling from T cell Receptor (TCR) interactions with peptide-bound major histocompatibility complex (MHC) and by CD28 interaction with the B7 ligands (CD80 or CD86) (Fig 1). T cell expression of CTLA4 is upregulated upon activation, allowing it to compete with B7 for CD28 binding. This competition provides a negative feedback loop that suppresses further T cell activation. PD-1 is also expressed on activated T cells (Fig 1). In the periphery, PD-1 binds to the receptors PD-L1 or PD-L2 expressed on healthy cells. This interaction results in immunosuppressive signaling in the T cell through the SHP-2 pathway.
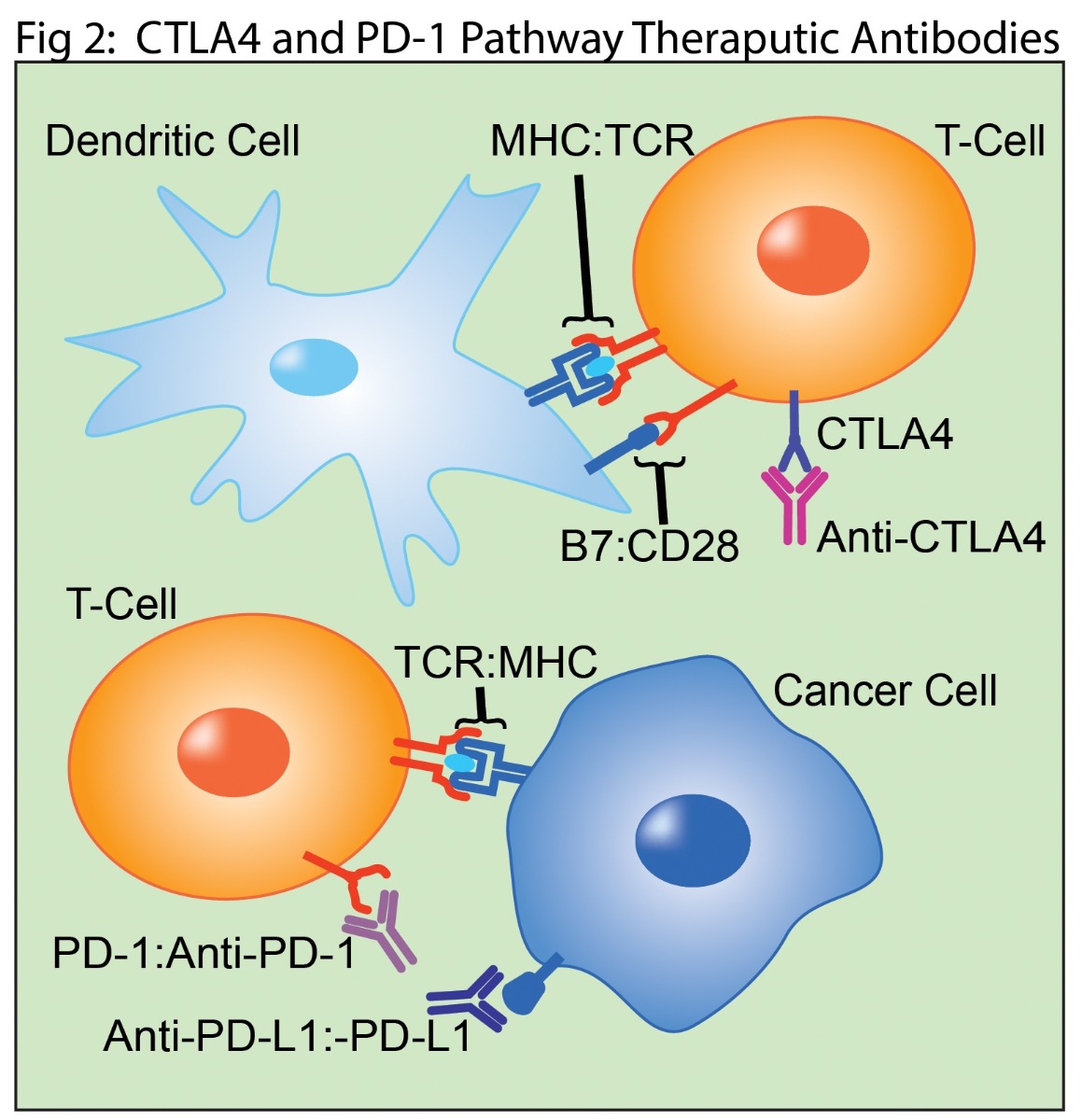
Therapeutic antibodies targeting the CTLA4 and PD-1 immune checkpoint pathways are currently the most successful monotherapies. CTLA4 was the first clinically successful checkpoint target (4). Inhibiting its interaction with B7 increases and prolongs T cell responses (Fig 2). PD-1-based treatments target T cell interactions with cancer cells, which frequently overexpress the PD-L1 and PD-L2 proteins (Fig 2) (4). Antibodies targeting the PD-1 pathway prevent extend the T cell response by preventing binding to its ligands. More recent strategies to take advantage of antibody effector functions targeting cancer cells for antibody mediated destruction. While these approaches have shown remarkable results in some patients, it remains true that the majority of cancer patients are either unresponsive to CTLA4 or PD-1 based monotherapies or will develop disease resistance.
Combining therapeutic antibodies targeting CTLA4 and PD-1 pathways enhance efficacy and have received FDA approval for treatment of metastatic melanoma. However, these targets only represent a fraction of immunomodulating receptors (4). Immune suppressive inhibitors including TIGIT, LAG3, TIM3, VISTA, and BTLA are well known as targets. The recent discovery of FGL1 as a potential new ligand for the immunosuppressive receptor LAG3 is generating particular excitement in preclinical studies (5). Synergistic approaches with immune stimulators including CD137, CD27, OX40, GITR, and HVEM also show great promise (3). These next generation receptors are being actively developed in early stage trials for the development of combination cancer treatments.
Small molecule therapeutics are often considered as co-therapies when combined with checkpoint inhibitors. Protein phosphorylation is central to cellular regulation and kinases are among the most common drug targets. Several kinases are under consideration in clinical trials as targets in combination therapies. This includes apatinib which targets the tyrosine kinase VEGFR (6) and combinations of dabrafenib and trametinib targeting BRAF and MEK in combination with anti-PD-1 (7).
Small molecule co-therapies are not limited to kinase inhibitors. Poly (ADP-ribose) polymerase (PARP) inhibitors have emerged as promising treatments for ovarian and breast cancer, most notably cancers for tumors with BRCA1/2 mutations (8). However, toxicities associated with PARP inhibition are linked to PD-L1 expression (8). This relationship provides the rational for clinical studies combining PARP inhibition with PD-1 and PD-L1-targeting treatments (9).
Similarly, Entinostat, is a selective HDAC1 and HDAC3 inhibitor that is also demonstrating promise as a combination therapy. Entinostat down-regulates immunosuppressive cell types in the tumor microenvironment, and results of stage II clinical trials using entinostat with anti-PD-1 in patients with non-small lung cancer who had been previously treated with anti-PD-1 therapy are encouraging (10). Such trials will increase understanding of epigenetic regulation and immunotherapy while uncovering the associated risks.
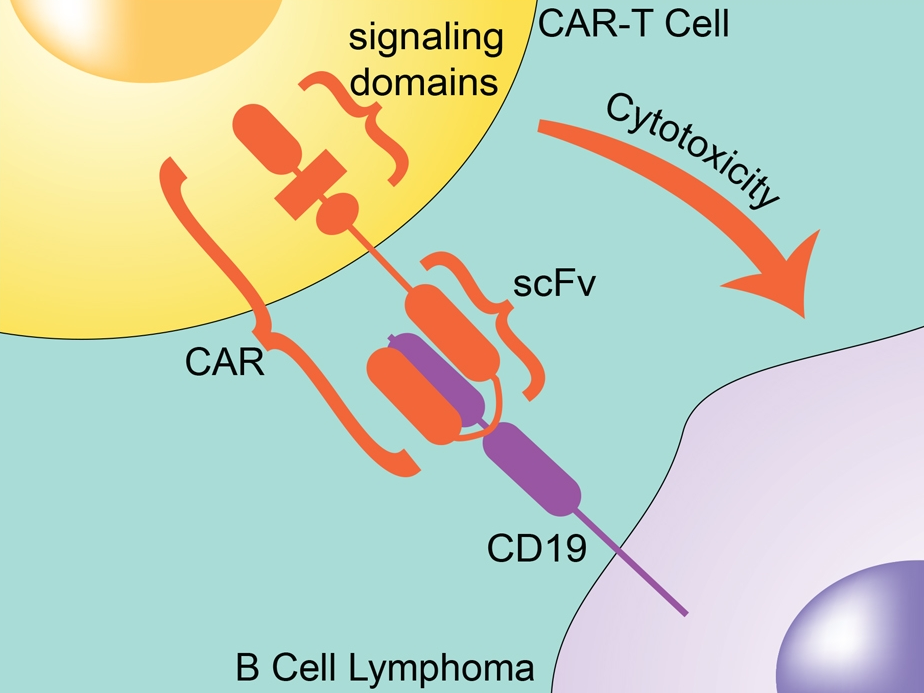
Adoptive cell transfer techniques with particular focus on CAR-T cells are an extremely interesting frontier in co-therapies for immune checkpoint inhibitors. A therapeutic chimeric antigen receptor, CAR, is a transmembrane protein designed to target proteins on cancer cells and signal for an immune response against the cancer. A patient’s own T cells are transfected with a gene expressing the CAR, creating personalized immune cells designed to treat the patient’s cancer.
The first CAR-T cells with FDA approval targeted CD19 to treat blood cancers. These initial CAR-T studies showed impressive results, especially with patients whose cancer was resistant to chemotherapy or had relapsed. Success with these CAR-T cells has inspired research on targets for both blood cancer and solid tumors. CD19-targeting CAR-T cells are also in development as co-therapies with therapeutic antibodies targeting the PD-1:PD-L1 and CD28:CTLA4 pathways (11).
At BPS Bioscience we strive to enhance drug discovery in immunotherapy. Our research tools can aid in finding drug candidates with higher potency and specificity. Such improvements are key to more effective and less toxic cancer treatments.
Contact BPS today to find out how we can help you meet your research goals.
References
10. ENCORE-601.