Targeting BCMA in Mutliple Myeloma
Multiple Myeloma
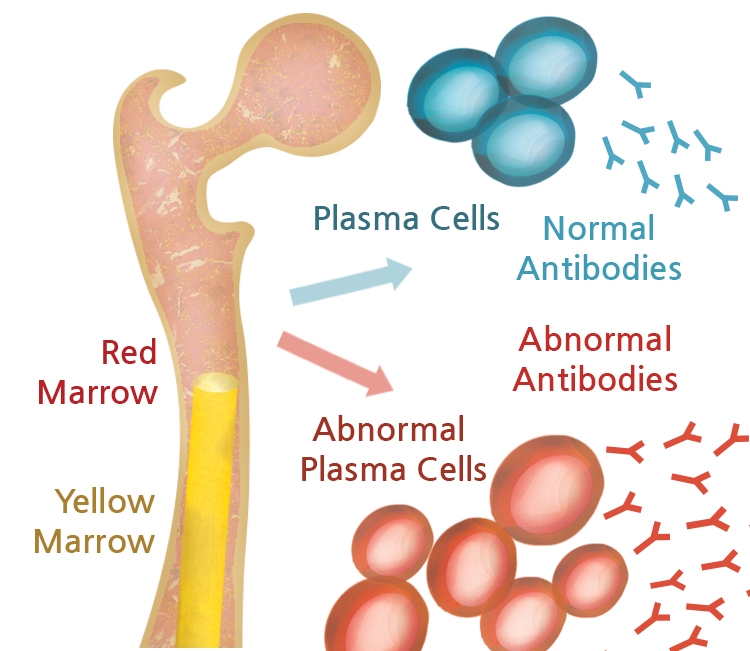
Multiple myeloma is the second most commonly diagnosed blood cancer, after non-Hodgkin lymphoma. Multiple myeloma is a cancer in which immortalized plasma cells accumulate in the bone marrow, causing severe pain, anemia, and kidney failure. Multiple myeloma was once considered universally fatal.
Improved diagnostic methods and innovative treatments are improving patient outcomes. Still, nearly all patients eventually relapse and become unresponsive to treatment, and the average 5 year survival rate is only about 50% (1).
There are two FDA-approved immunotherapies for relapsed multiple myeloma—these treatments are monoclonal antibodies targeting CD38 (daratumumab) or SLAMF7 (elotuzumab). Unfortunately daratumumab also interferes with blood panel testing (2) and induces loss of CD38 on red blood cells (3). Similarly, SLAMF7 is also present on natural killer (NK) cells, T cells, and B cells, and kills off lymphocytes expressing high levels of SLAMF7 (4).
BCMA, BAFF, & APRIL
BCMA [B Cell Maturation Antigen, also known as tumor necrosis factor receptor superfamily member 17 (TNFRSF17)], is an interesting drug target for multiple myeloma. This transmembrane receptor is naturally expressed on plasma cells and is consistently expressed at high levels on malignant plasma cells. Otherwise, BCMA exhibits very restricted expression in normal tissues. BCMA is selectively induced during plasma cell differentiation and is nearly absent on naive and memory B cells (5).
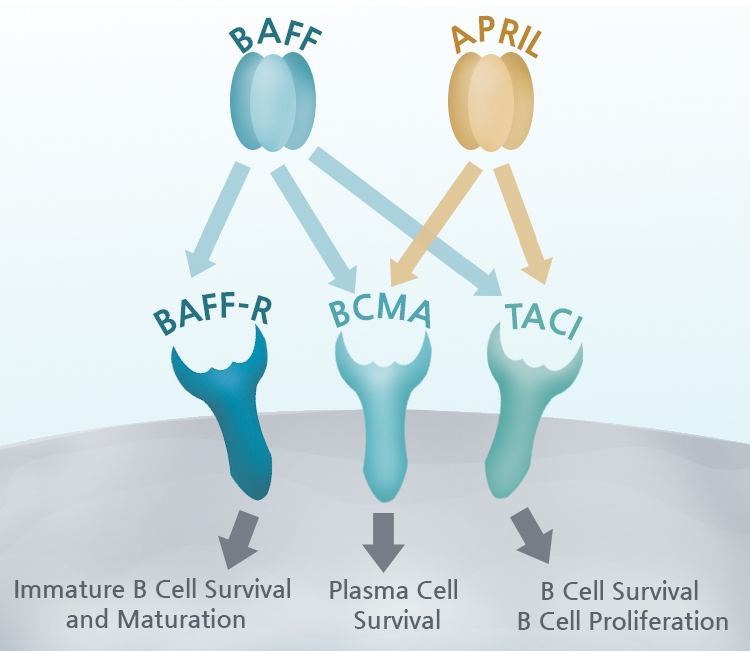
BCMA functions by binding to BAFF (B-cell activating factor/TALL-1/CD257/BLyS), a cytokine expressed by B cell lineage cells, and leads to activation of NF-kB and the MAPK8/JNK signaling pathway. Signaling through BAFF and BCMA stimulates plasma cells to undergo proliferation and to maintain long-term humoral immunity and survival. BAFF is required for homeostasis and maintaining normal B-cell development, but it also promotes the survival of malignant B cells.
There are two other receptors for BAFF besides BCMA: BAFF Receptor (BAFF-R) triggers naïve B cell survival and maturation, while TACI (Transmembrane activator and CAML interactor) regulates B cell antibody responses, isotype switching, and homeostasis (6). Complicating matters, BCMA can bind at high affinity to a related protein, APRIL (A proliferation-inducing ligand), which is involved in B cell development and autoimmune response.
The complex interactions between BCMA and its ligands BAFF and APRIL are key to the maintenance and survival of plasma cells, but BCMA also has been linked to a number of cancers, autoimmune, and infectious diseases that suggest additional roles for BCMA activity.
Clinical Trials
Not surprisingly, multiple BCMA-targeted therapies are under development, especially following impressive anti-myeloma clinical responses in relapsed multiple myeloma patients using the first chimeric antigen receptor (CAR) T cell therapy (7).
Importantly, these are patients who had already failed at least three prior lines of therapy, including anti-CD38 antibody, a proteasome inhibitor, and an immunomodulator. In December 2018, Janssen and Legend Biotech completed a phase 1/2 clinical trial with their own BCMA-directed CAR T cell therapy, LCAR-B38M. The results were exceptional, with 88% of patients showing a positive response, and 74% demonstrating a complete response to the treatment, with no signs of cancer (8).
Other approaches include GlaxoSmithKline's GSK2857916, a novel anti-BCMA antibody conjugated to the drug auristatin F. GSK2857916 induced significant clinical responses, killing myeloma cells even when expression levels of BCMA were low (9). Celgene, Amgen, Novartis, Poseida, Nanjing, and other companies are all pursuing BCMA-based immunotherapy strategies, include bispecific T-cell engagers, bi- or trispecific antibodies, and improved forms of next generation CAR T.
Products
BPS Bioscience remains at the forefront of BCMA-related research. Contact us today to learn more about the ways BPS Bioscience can provide the tools and expertise to help you fulfill your research goals.
References
- www.cancer.net/cancer-types/multiple-myeloma/statistics
- Chapuy, CI., et al. Transfusion. 2015 Jun;55(6 Pt 2):1545-54. doi: 10.1111/trf.13069.
- Sullivan, HC., et al. Blood. 2017 Jun 1;129(22):3033-3037. doi: 10.1182/blood-2016-11-749432
- Gogishvili, T., et al. Blood 2017: blood-2017-04-778423; doi: https://doi.org/10.1182/blood-2017-04-778423
- Khattar, P., et al. Blood 2017 130:2755
- Mackay F, Schneider P. Cytokine Growth Factor Rev. 2008 Jun-Aug;19(3-4):263-76. doi: 10.1016/j.cytogfr.2008.04.006.
- Ali SA, et al. Blood (2016) 128(13):1688–700.10.1182/blood-2016-04-71190
- Al Idris, A. 2018. https://www.fiercebiotech.com/biotech/ash18-j-j-legend-car-t-treatment-posts-88-orr-multiple-myeloma
- Cho, S.F., et al. Front Immunol. 2018; 9: 1821.
Proteins
- BAFF, His-Avi-Tag - #100194
- BAFF, unlabeled - #90100
- Human BAFF-R(CD268) - #90103
- BCMA, Fc-fusion, Avi-Tag - #79465
- BCMA, Fc-fusion (IgG1), Avi-Tag, Biotin-Labeled - #79467
Assay Kits
Cell Lines
Antibody