PROTAC-Driven Ubiquitination Assay Kit for BET Bromodomains
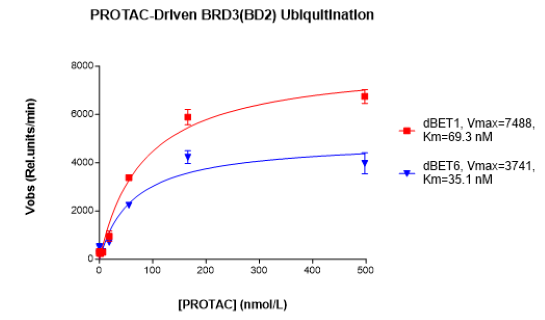
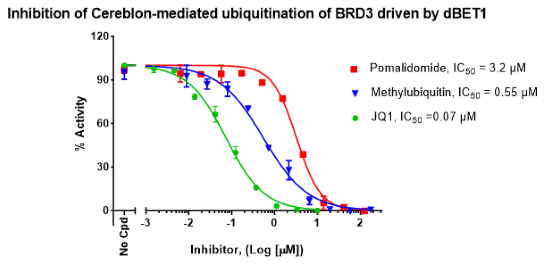
The PROTAC-Driven Ubiquitination Assay Kit for BET Bromodomains is designed for the testing and profiling of PROTACs targeting the bromodomains on the BET (bromodomain and extraterminal) protein family. After tertiary complex formation between the E3 protein, the PROTAC and the protein of interest, ubiquitination of the target protein is a critical step preceding its degradation. The PROTAC-Driven Ubiquitination Assay Kit for BET Bromodomains comes in an AlphaLISA® format with enough optimized assay buffer, purified recombinant E1 (UBE1), E2 (UbcH5b), and E3 enzymes (Cereblon complex), BRD3 (BD2) (bromodomain-containing protein 3) (amino acids 305-417) as target protein, ATP, and biotinylated ubiquitin for 384 reactions. This kit also contains the PROTAC dBET1 as the control, and JQ1 as control inhibitor.
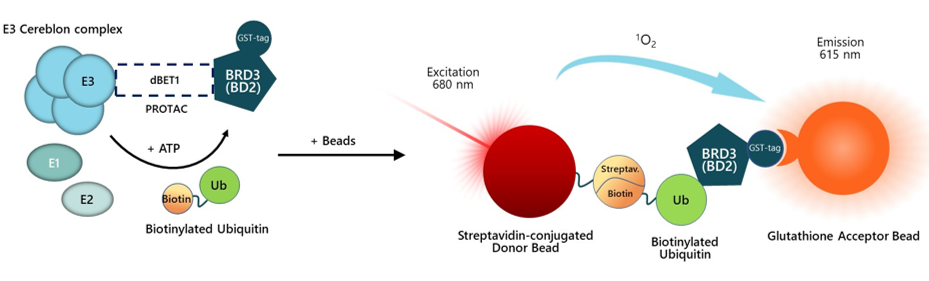
Figure 1. Schematic representation of the principle of the PROTAC-Driven Ubiquitination Assay Kit for BET Bromodomains.
dBET1 or a PROTAC® of interest is incubated with Cereblon complex and BRD3 (BD2), bringing them in close proximity. Ubiquitination reaction occurs following the addition of E2 enzyme, ATP, biotinylated ubiquitin, and, finally, E1 enzyme. Once ubiquitination has taken place, AlphaLISA® donor and acceptor beads are added. BRD3 (BD2) contains a GST-tag, which is recognized by GSH-AlphaLISA™ acceptor beads. Ubiquitinated BRD3 (BD2) contains biotin-ubiquitin, that binds to Streptavidin donor beads. Upon excitation of the donor bead, a singlet oxygen is generated. The singlet oxygen excites the acceptor bead, which emits light. The resulting signal is directly proportional to the level of ubiquitination of BRD3 (BD2).
Need us to run inhibitor screens or profile your compounds against PROTAC®-driven ubuitination for BET Bromodomain? Check out our Bromodomain Screening And Profiling or PROTACs ® & Protein Degradation Services.
- AlphaLISA GSH Acceptor Beads, 250 µg (PerkinElmer #AL109C)
- AlphaScreen Streptavidin-Conjugated Donor Beads, 5 mg/ml (PerkinElmer #6760002S)
- Optiplate 384 (PerkinElmer #6007290)
- AlphaScreen microplate reader
- Adjustable micropipettor and sterile tips
| Catalog # | Name | Amount | Storage |
| 80301 | UBE1 (UBA1), FLAG-Tag* | 25 µg | -80°C |
| 80314 | UbcH5b, His-Tag (Human)* | 60 µg | -80°C |
| 100329 | Cereblon/DDB1/Cul4A/ Rbx1 Complex* | 60 µg | -80°C |
| 31033 | BRD3 (BD2), GST-Tag* | 5 µg | -80°C |
| dBET1 (MW=785 Da) | 2 x 0.31 µg | -20°C | |
| 27403 | 10 mM (+)-JQ1 | 10 µl | -20°C |
| U2 Assay Buffer | 10 ml | -20°C | |
| 10 mM ATP | 400 µl | -80°C | |
| Biotin-Ubiquitin | 400 µl | -80°C | |
| Plate sealer | 1 | Room Temp |
*The initial concentration of enzymes is lot-specific and will be indicated on the tube containing the protein.
Cereblon is the substrate-binding component of the E3 protein ligase complex Cereblon-CUL4A (cullin-4A)-RBX1 (RING-box protein 1) that targets proteins for degradation through the proteasome system. The binding of Cereblon to a substrate protein engages the E3 ligase activity of the complex and results in the ubiquitination and ultimate degradation of the substrate protein. PROTACs are bifunctional molecules that bring together a therapeutic target and an E3 ligase to promote the ubiquitination and ultimately the degradation of the target. Efficiency of ubiquitination is an important factor for optimization of heterobifunctional degraders. BRD3, also known as RING3L (RING3-like protein) belongs to the BET family of proteins and is involved in transcription by associating with acetylated lysines present in histones and transcription factors. Chromosomal translocations of BRD3 with NUT (nuclear protein in testis) can lead to cancer, and it has been shown that the use of BET inhibitors or depletion of BRD3 can slow down cancer progression in models of prostate cancer. The use of PROTAC technology for degradation of BRD proteins is an active field of research. Development of novel PROTAC molecules involved in the recruitment of BRD3 (BD2) provides a framework for future efforts to utilize Cereblon to degrade other proteins of interest.
Kaur, S.D., et al. 2023 Cancer Lett; 556: 216065.
Wang C., et al., 2022 J Enzyme Inhib Med Chem 37(1): 1694-1703.