PRMT5/MEP50 Methylation
Comparing Methylation Patterns of Recombinant PRMTs: Abnormal PRMT5/MEP50 Methylation
PRMTs – Roles & Mechanisms
Protein arginine methyltransferase 5 (PRMT5) is a critical regulatory protein linked to genome organization, cell cycle regulation, and stem cell differentiation1. It forms an active complex with MEP50 and transfers methyl groups from S-adenosylmethionine (SAM) to histone proteins, transcription factors, and other regulatory proteins. PRMT5 is a type II PRMT enzyme, meaning that these methylation events form symmetrically di-methylated arginine residues—with mono-methylated arginine as an intermediary. On the other hand, type I PRMT enzymes mediate asymmetric di-methylated arginine residues. This category includes PRMT1, PRMT3, PRMT6, and PRMT8. Generally, asymmetrically methylated Arginine residues activate gene expression, while symmetrically methylated arginine residues repress gene expression2.
Methylation Patterns of PRMT5
Recombinant PRMT5/MEP50 Complex (HEK-293 derived) (#51045) was found to have mono-methylation and symmetric di-methylation activity in addition to an abnormal asymmetric di-methylation activity. These activities were detected using in vitro biochemical ELISA-based assays (#52002L). Initially, primary antibody specificity was determined by coating Mono-Methylated Arginine3 H4, 1-21[Biotinylated] (H4 R3Me1), symmetric Di-Methylated Arginine3 H4, 1-21[Biotinylated] (H4 R3Me2sym) (#52150-3), or asymmetric Di-Methylated Arginine3 H4, 1-21[Biotinylated] (H4 R3Me2asym) (#52150) onto a Neutravidin plate (#79512). This was followed by detection using three methylation-state primary antibodies for the above ligands. Luminescence was developed by addition of secondary anti-Rabbit HRP-labeled antibody followed by addition of HRP. All 3 primary antibodies (available for purchase upon request) showed no non-specific binding (figures 1, 2 and 3 below).
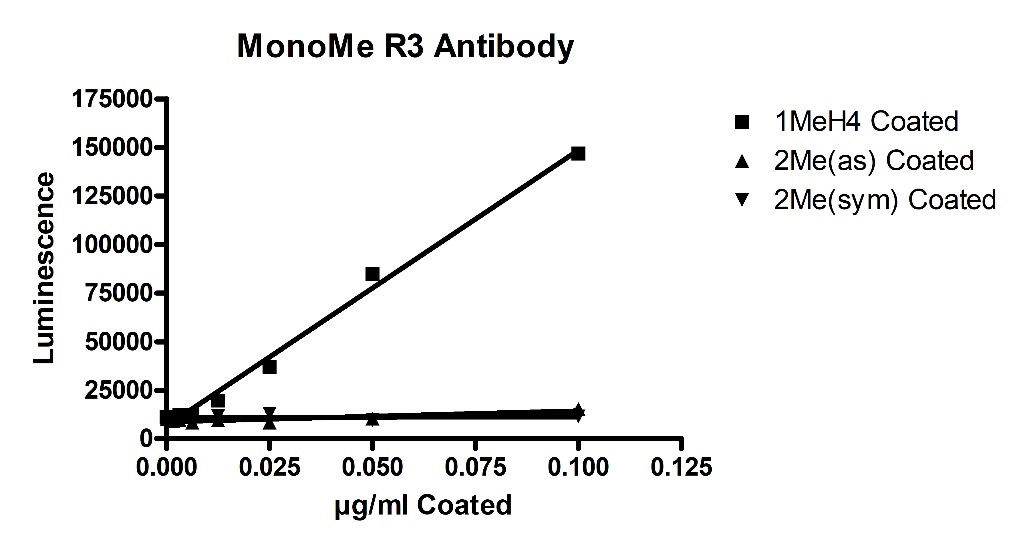
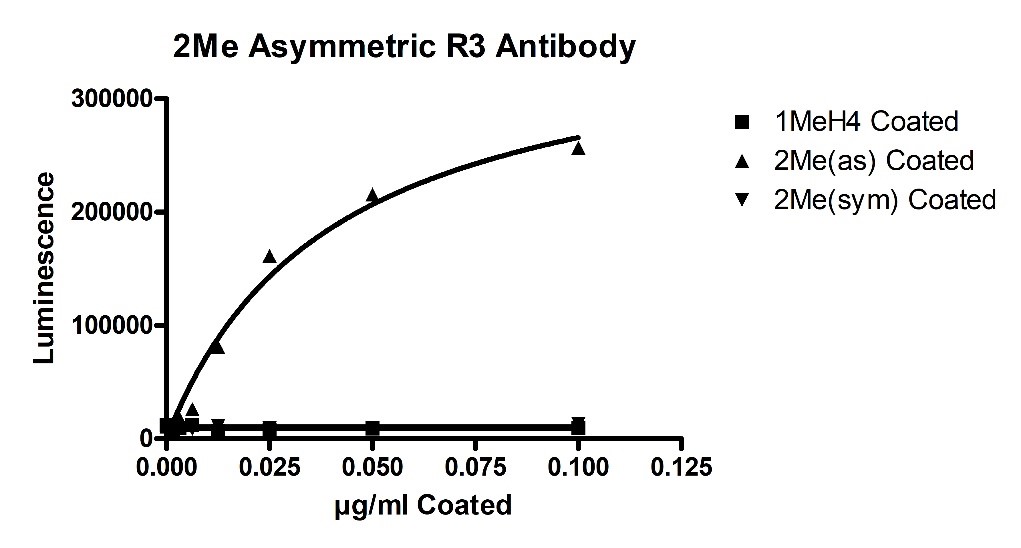
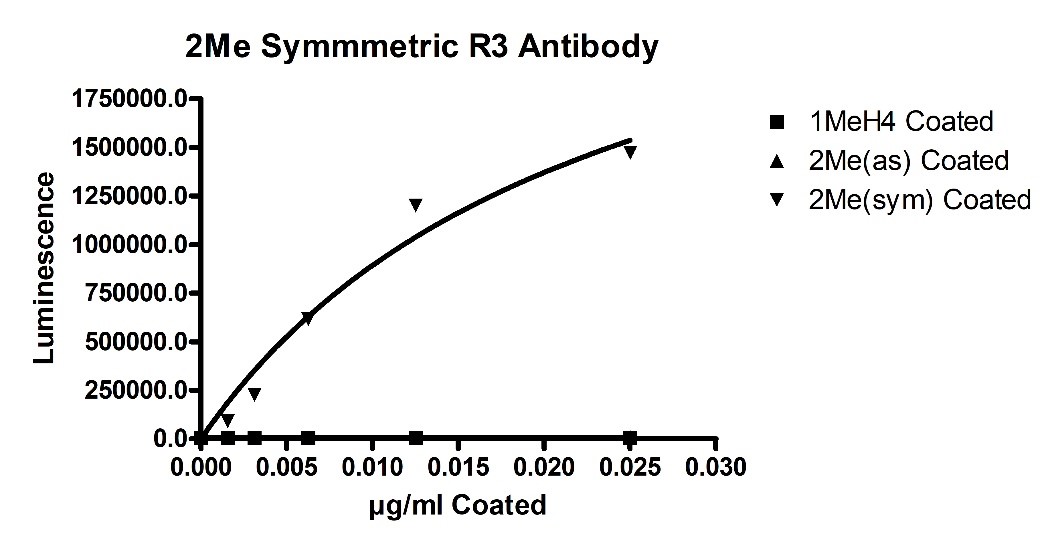
Figure 1-Mono-methylated Histone H4 Antibody Specificity
Figure 2- Asymmetric Di-methylated Histone H4 Antibody Specificity
Figure 3 - Symmetric Di-methylated Histone H4 Antibody Specificity
PRMT5/MEP50 Activity
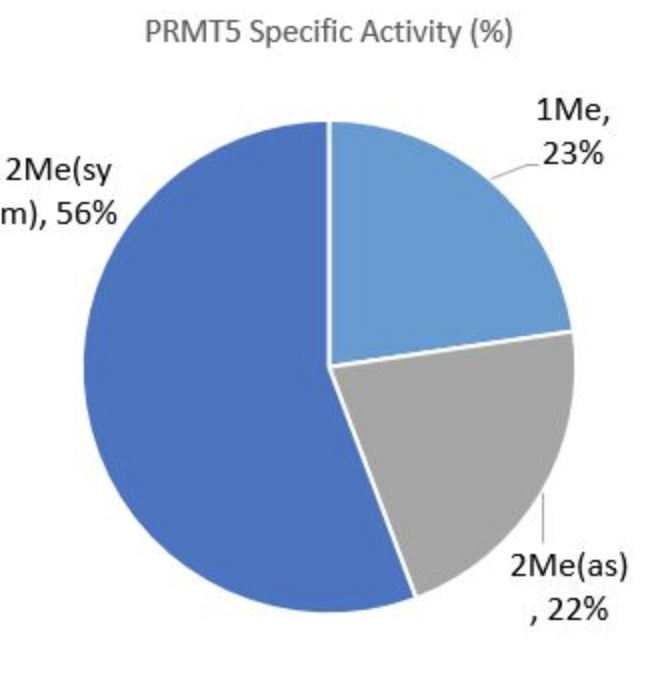
To determine PRMT5/MEP50 activity, unmodified H4, 1-21[Biotinylated] was coated onto a Neutravidin plate. Next, various amounts of recombinant PRMT5/MEP50 was incubated for 1 hour with 40 µM S-adenosylmethionine (#52120). This was followed by a 1 hour incubation with one of the three antibodies described above, and a 30 minute incubation with Secondary HRP-labeled antibody. All three methylation products were detected, with the R3Me2sym state being the most prominent. Specific activity (SA) was determined using pre-coated methylated standards. The percentages below are based on total activity of the three products being detected.
Figure 4-PRMT5/MEP50 Specificity. H4 R3Me1 SA = 0.023 pmole/min/µg. H4 R3Me2(sym) SA = 0.057 pmole/min/µg. H4 R3Me2(asym) SA = 0.022 pmole/min/µg
Methylation Patterns of Type I PRMTs
This trend of abnormal methylation was not seen in other recombinant PRMT enzymes. Type I PRMTs—PRMT1 (#51040), PRMT3 (#51043), PRMT6 (#51049) and PRMT8 (#51052)—produced only asymmetric di-methylated arginine residues and the intermediate mono-methylated arginine. When following the same protocol as above, primarily only H4 R3Me2(asym) was detected. PRMT3 is less active than other PRMTs and thus produced more of the H4 R3Me1 product. Longer incubation times and increased enzyme concentration produces more of the H4 R3Me2(asym) product.
Figure 5-PRMT1, PRMT6, PRMT8 Specificity for Histone H4
Biological Relevance
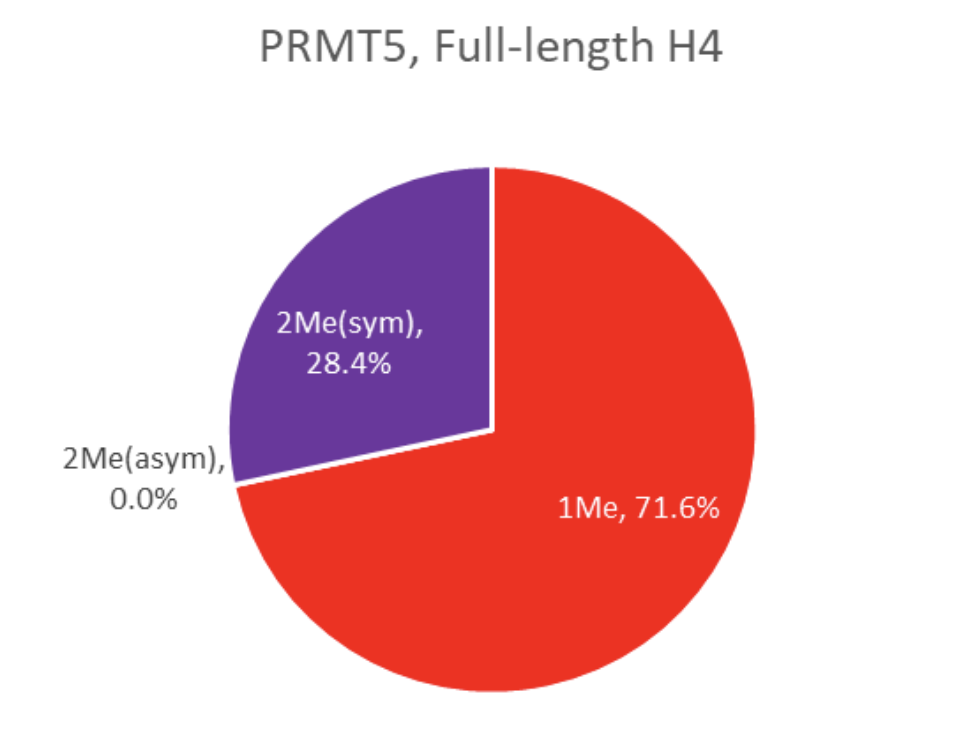 To determine biological relevance, full length Histone H4 (#52024) was coated onto a MaxiSorp™ plate overnight. The same protocol as described above was followed and specific activity percentages were determined (figure 5). This result indicates that the abnormal methylation activity described in figure 4 is only seen with a truncated histone peptide. In BPS Bioscience’s PRMT Chemiluminescent assay kits, Histone H4, 1-21[Biotinylated] is used as the coated substrate.
To determine biological relevance, full length Histone H4 (#52024) was coated onto a MaxiSorp™ plate overnight. The same protocol as described above was followed and specific activity percentages were determined (figure 5). This result indicates that the abnormal methylation activity described in figure 4 is only seen with a truncated histone peptide. In BPS Bioscience’s PRMT Chemiluminescent assay kits, Histone H4, 1-21[Biotinylated] is used as the coated substrate.
Figure 6-PRMT5/MEP50 Specificity with full-length H4 as substrate
Conclusion
PRMT5 can initiate multiple methylation states in its target proteins, and it exhibits versatile involvement in a variety of protein-protein interactions. Among these is the ability to mediate gene expression, prompting cell growth or inducing apoptosis. This makes PRMT5 a very promising target of interest for both epigenetic and cancer therapeutics. See PRMT5 & Cancer.
References
[1] Stopa, N., Krebs, J. E., and Shechter, D. (2015). The PRMT5 arginine methyltransferase: many roles in development, cancer and beyond, Cellular and Molecular Life Sciences : CMLS 72, 2041-2059.
[2] Di Lorenzo, A., & Bedford, M. T. (2011). Histone arginine methylation. FEBS letters, 585(13), 2024–2031.