LAG3 as a Cotherapy for Immune Checkpoints
Cotherapy with PD-1
Therapeutic antibodies targeting the immune checkpoint receptors PD-1 and CTLA4 substantially changed the prognosis for many cancer patients. When successful, these treatments extend lives. Still, cancer is relentless. Only a relatively small number of patients respond or have substantial remission when these treatments are used as monotherapies.
Much of the current research on immunomodulating receptors is aimed at finding combination therapies to overcome the limitations of targeting either PD-1 or CTLA4 alone. Given the large number of immunomodulatory receptors, it is not surprising that many promising targets are being investigated as possible sources of synergies for PD-1- or CTLA4-based treatments.
Lymphocte-activation gene 3 (LAG-3) is an immunosuppressive receptor that shows promise in early clinical trials as a target for cancer immunotherapy(1). It was first identified as a receptor expressed on activated CD4+ and CD8+ T-cells and natural killer cells(2). In early cellular and animal studies, LAG-3 was associated with the suppression of T-cell activation through linkage to cytokine production(3). Coexpression of immunosuppressive receptors contributes to T-cell exhaustion characterized by reduced T-cell proliferation, cytokine production, and low levels of cytotoxicity(4). Coexpression of PD-1 and LAG-3 in viral models of exhaustion provided the first indication that LAG-3 had potential as a cotherapy for PD-1 based treatments.(5)
LAG3 Ligands
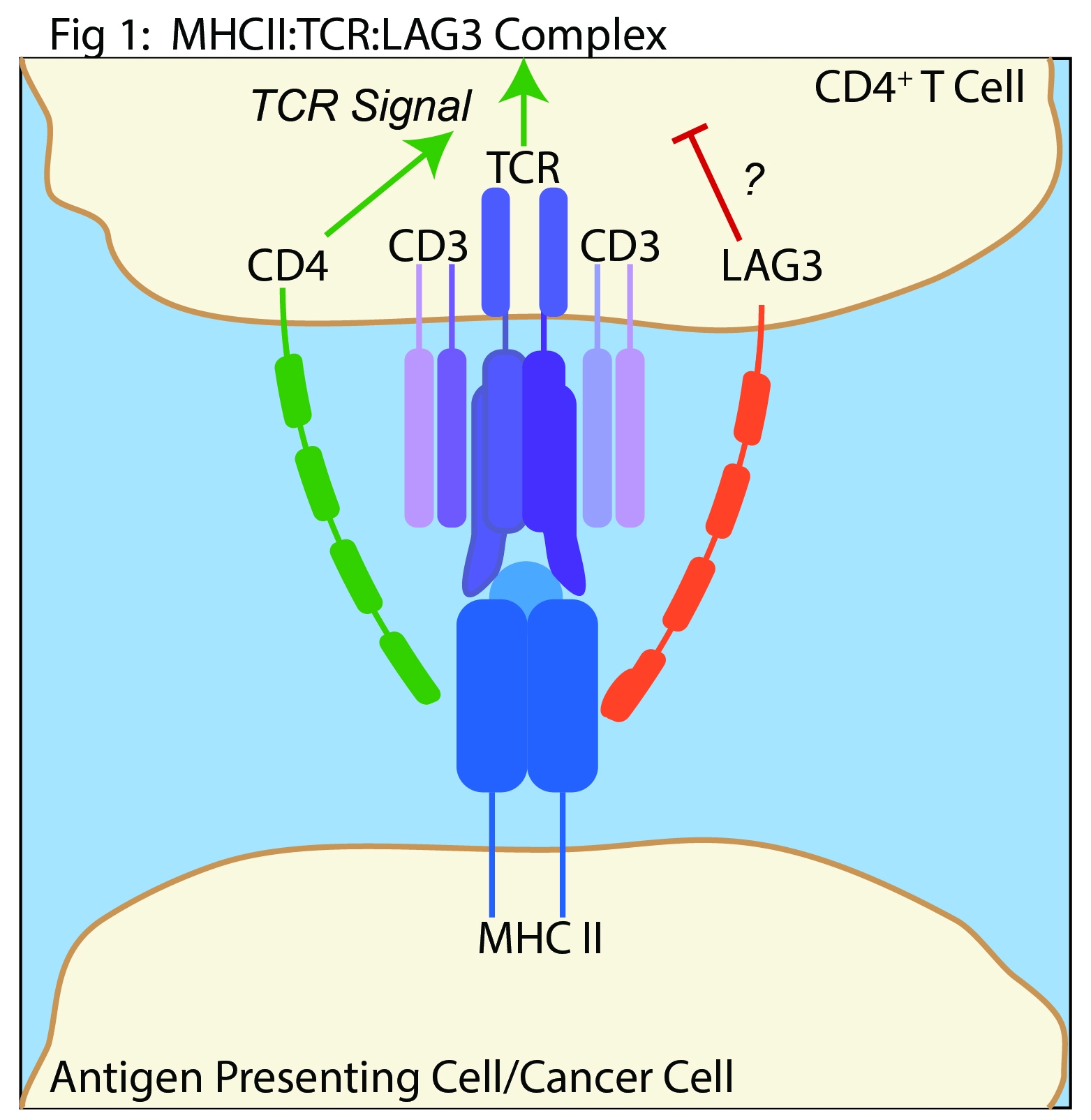
Understanding the mechanism of receptor function is vital for the design and development of therapeutic strategies. A critical step is identifying the ligands that control receptor activity. MHC class II molecules were the first ligands reported for LAG-3 using cell clustering assays and blocking by antibodies against LAG-3 and MHC II(1). Since its initial discovery, this interaction has been thoroughly investigated in cell-based and animal models. These studies include estimates of binding affinity and mapping of LAG-3 residues that contribute to the interaction with MHC II(6, 7).
The role of MHC II in LAG-3 mediated control of immunity is highly debated. LAG3 is structurally analogous to CD4 and is believed to oppose CD4 function(1) [Fig 1]. However, LAG-3 suppresses the function of CD8+ T cells and natural killer cells(8). Neither of these cell types interact with MHC II. Furthermore, multiple LAG-3 specific antibodies that do not inhibit MHC II binding can stimulate T cell activation and anti-tumor activity(9). Finally, the mechanism of LAG-3 signaling through MHC II binding is not well understood. The cytoplasmic domain of LAG-3 that mediates T cell activity through a MHC II-dependent mechanism is not thoroughly defined.
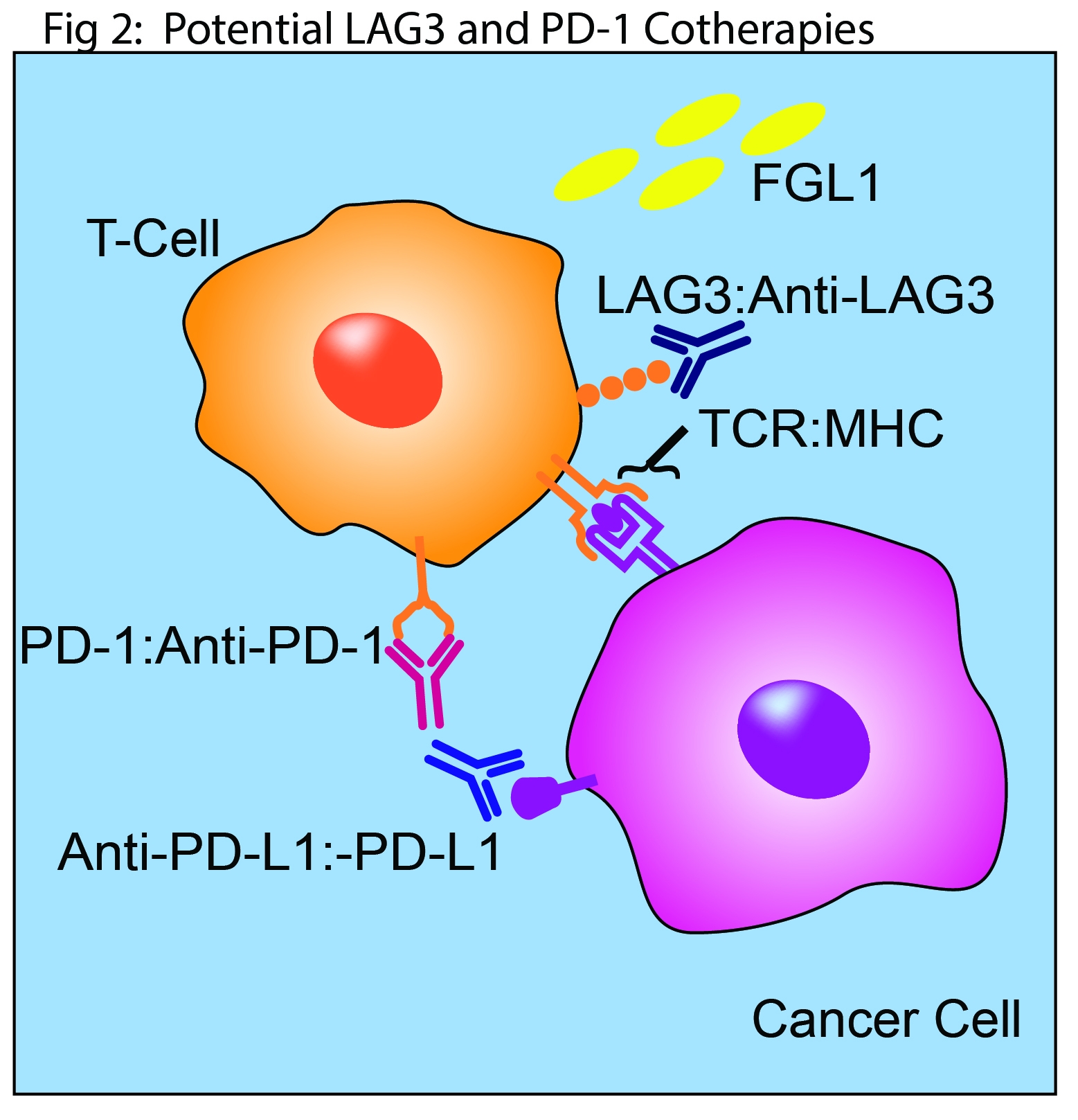
The recent discovery of Fibrinogen-like Protein-1 as a ligand of LAG-3 is a potential breakthrough(10). FGL1 is secreted from liver cells and is associated with metabolic functions; however, a role in immune regulation was not previously known. FGL1 was identified as a LAG-3 ligand in cell-based studies and its binding to LAG-3 through a MHC II independent mechanism was confirmed by biochemical methods. In the same study, cell-based methods demonstrated the FGL1 interaction with LAG3 suppresses cytokine production in T cells, and silencing of FGL1 is tumor inhibiting in mouse models. Finally, data analysis of Oncomine data bases indicate FGL1 is overexpressed in many human cancers.
Multiple anti-LAG3 monoclonal antibodies that block interaction with MHC II are in early clinical trials both as cancer monotherapies and in combination with anti-PD1 inhibitors(1). This mechanism of action may be vital to their eventual clinical success. However, the early results with FGL1 highlight the potential for other pursuit of other strategies in the design and development of next generation clinical candidates [Fig. 2]. The identification of FGL1 as a ligand for LAG3 is likely to energize studies of other alternative ligands. These include the lectins galectin-3 and LSECtin which are implicated in T cell regulation, and α-synuclein which is associated with Parkinson’s disease(1).
References
- L. P. Andrews, A. E. Marciscano, C. G. Drake, D. A. Vignali, LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev 276, 80-96 (2017).
- F. Triebel et al., LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med 171, 1393-1405 (1990).
- B. Huard, M. Tournier, T. Hercend, F. Triebel, F. Faure, Lymphocyte-activation gene 3/major histocompatibility complex class II interaction modulates the antigenic response of CD4+ T lymphocytes. Eur J Immunol 24, 3216-3221 (1994).
- S. H. Baumeister, G. J. Freeman, G. Dranoff, A. H. Sharpe, Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol 34, 539-573 (2016).
- S. D. Blackburn et al., Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 10, 29-37 (2009).
- B. Huard et al., Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proc Natl Acad Sci U S A 94, 5744-5749 (1997).
- B. Huard, P. Prigent, M. Tournier, D. Bruniquel, F. Triebel, CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur J Immunol 25, 2718-2721 (1995).
- A. C. Anderson, N. Joller, V. K. Kuchroo, Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 44, 989-1004 (2016).
- S. Z. Cemerski, S. Chenard, M. Laskey, J. Cui, L. Shukla, R. Haines, B. Hsieh, E. Beaumont, M. Mattson, J. Blumenschein, W. Hirsch, H. Fayadat-Dilman, L. Liang, L. Malefyt, R. D. W., T cell activation and anit-tumor effacay of anti-LAG-3 antibodis is independent of LAG-3-MHCII blocking capacity. J. Immunother. Cancer 3, (2015).
- J. Wang et al., Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 176, 334-347 e312 (2019).