KRAS (G12D), Isoform A, His-Tag, GppNHp-Loaded Recombinant
Recombinant human KRAS (KRAS proto-oncogene GTPase), isoform a, encompassing amino acids 2-186(end). This construct contains an N-terminal His-tag (6xHis) followed by an optimal TEV protease target sequence (TEV: tobacco etch virus cysteine protease BPS Bioscience #50308). The protein also contains mutation of interest G12D. The protein was affinity purified and loaded with GppNHp, a non-hydrolyzable GTP analog. Unbound GppNHp was removed by spin column. Ready for use in KRAS-RAF binding studies or inhibitor assays
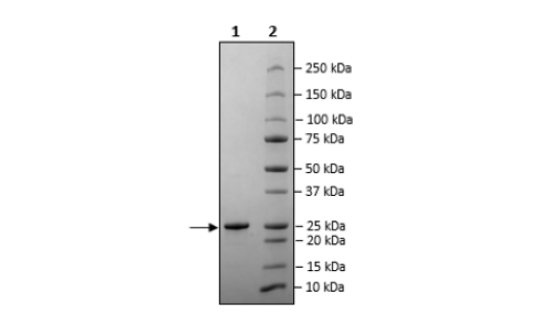
≥90%
Aqueous buffer solution
20 mM HEPES, pH 7.4, 150 mM NaCl, and 1 mM DTT
RAS mutations are responsible for more than 30% of human cancers with KRAS(G12D) being one of the KRAS mutations that is found frequently in pancreatic and colon cancers. Recent studies have led to the discovery of small molecules, such as MRTX-1133, able to lock KRAS conformation in the inactive GDP-bound state, thereby blocking the KRAS(G12D)-mediated signaling pathway. The development of compounds that affect the nucleotide exchange (GDP to GTP) reaction in KRAS is one of the approaches that may inhibit tumor cell growth in KRAS(G12D)-driven tumors.
The KRAS (Kirsten rat sarcoma virus) gene is subject to alternative splicing, resulting in two isoforms: KRAS-A and KRAS-B. These isoforms differ by amino acids 151, 153, 165, and 166 and within the hypervariable region (amino acids 167-189). KRAS-B contains a long polybasic stretch, while KRAS-A has a shorter polybasic region with a palmitoylation site. These differences confer distinct biological characteristics to the two isoforms. When studying a mutant of KRAS, it is important to know which isoform is being studied to make sure that the correct wild-type isoform is used for comparison. The identification of new strategies targeting KRAS G12V will bring large benefits in cancer therapy.