Dominant Negative TGF-β Receptor Type II (TGF-βRII) Lentivirus
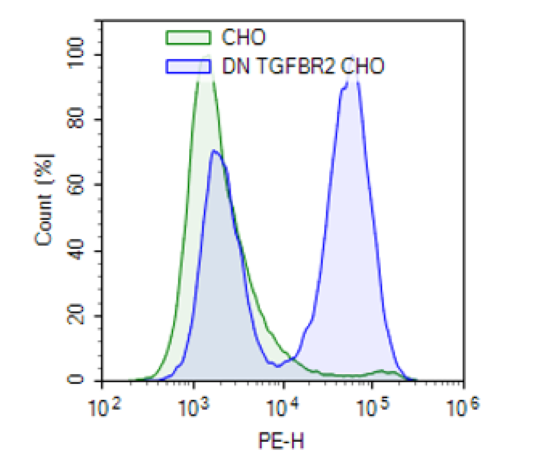
Dominant Negative TGF-β Receptor Type II (TGF-βRII) Lentivirus are replication incompetent, HIV-based, VSV-G pseudotyped lentiviral particles ready to transduce nearly all types of mammalian cells, including primary and non-dividing cells. These viruses result in expression of human dominant negative TGF-βRII, missing the intracellular kinase domain (NM_003242.6; amino acid 1-191), driven by an EF1a promoter and a puromycin selection marker (Figure 1).
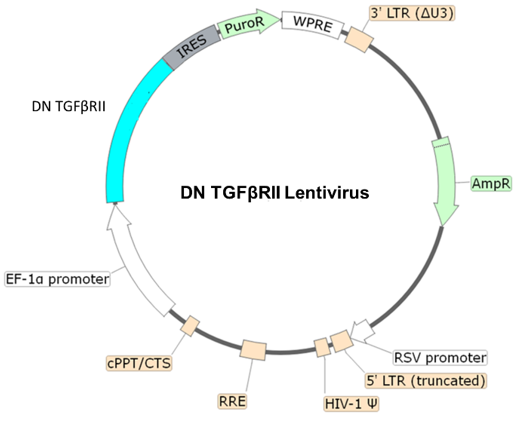
Figure 1. Schematic of the lenti-vector used to generate Dominant Negative TGF-β Receptor Type II (TGF-βRII) Lentivirus
The lentivirus particles were produced in HEK293T cells in medium containing 90% DMEM + 10% FBS. Virus particles can be packaged in custom formulations and produced at higher titers by special request, for an additional fee.
Transforming growth factor receptor beta 2 (TGFBR2), or TGFβRII, encodes the TGFβ receptor serine/threonine kinase, which is a transmembrane protein that forms a heterodimeric complex with other receptor proteins and binds TGFβ. The association of TGFβ with TGFβRII leads to the phosphorylation of proteins, such as SMADs, involved in cell proliferation, cell cycle arrest, wound healing, and immunosuppression. Dysfunction of the TFGβ signaling tends to result in cancer development and progression. In the case of solid tumors, TGFβ signaling plays a role in creating a highly immunosuppressive TME (tumor microenvironment), restricting the efficacy of CAR (chimeric receptor antigen)- T cells, which have proved successful in the treatment of hematological cancers. Recently, the use of CAR-T cells armored with a dominant negative form of TGFβRII, missing the intracellular kinase domain, for the treatment of prostate cancer resulted in promising outcomes. 5 out of 13 patients did suffer cytokine release syndrome, which continues to be a concern with CAR-T applications, but on the whole the use of a dominant negative TGFβ receptor to armor CAR-T cells appears to be an approach that deserves further attention in the cancer therapy field.
Ikushima H. and Miyazono K., 2010 Nature Reviews Cancer 10:415-424.
Narayan V., et al., 2022 Nat Med 28:724-34.