Ubi-Trans™ UBA6-UbcH5b TR-FRET Assay Kit
The Ubi-Trans™ UBA6-UbcH5b TR-FRET Assay Kit is a homogeneous, sensitive TR-FRET (Time-Resolved Fluorescence Resonance Energy Transfer) assay designed to measure UBA6 (ubiquitin-like modifier activating enzyme 6)-dependent transfer of Ub (ubiquitin) to UbcH5b (Ubiquitin-conjugating enzyme E2 D2) E2 enzyme. It utilizes a Terbium (Tb)-labeled donor and dye-labeled streptavidin acceptor to complete the TR-FRET pairing. The kit comes in a convenient 384-well format and contains enough purified UbcH5b, purified UBA6, Biotin-Ubiquitin, anti-His Tb (terbium)-labeled donor, dye-labeled streptavidin acceptor, and assay buffer for 400 reactions.
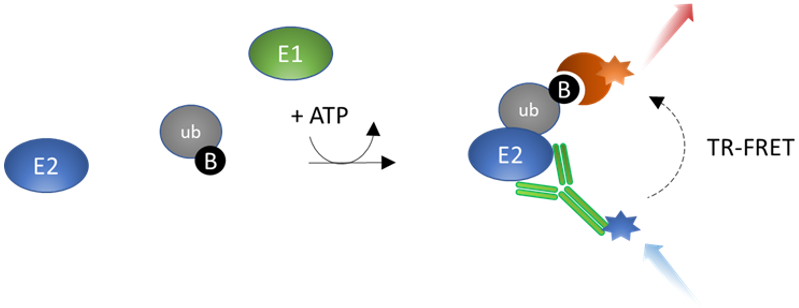
Figure 1: Ubi-Trans™ UBA6-UbcH5b TR-FRET Assay Kit schematic.
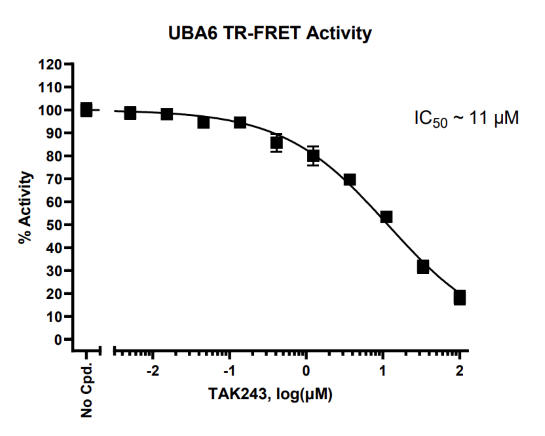
In the presence of ATP, UBE6 (E1) transfers biotin-conjugated ubiquitin to the substrate UbcH5b (E2). The Tb-labeled anti-His antibody binds to the His-tagged E2 conjugating protein, while the dye-labeled streptavidin acceptor binds to Biotin-Ubiquitin. The complex forms when ubiquitin is transferred to the E2 enzyme by E1. In the absence of ubiquitination, the complex does not form and energy transfer does not occur. When E2 is ubiquitinated the complex is formed, TR-FRET pairing is complete, and an increase in acceptor emission is observed. Thus, TR-FRET signal is proportional to UBE6 activity.
Need us to run inhibitor screens or profile your compounds against UBA6? Check out our Ubiquitination Screening Services.
- Fluorescent microplate reader capable of measuring Time Resolved Fluorescence Resonance Energy Transfer (TR-FRET)
- Adjustable micropipettor and sterile tips
- Rotating or rocker platform
| Catalog # | Name | Amount | Storage |
| 80303 | UBA6 (UBE1L2), FLAG-Tag* | 4.8 µg | -80°C |
| 80314 | UbcH5b, His-Tag (Human)* | 5 µg | -80°C |
| Biotin-Ubiquitin | 80 µl | -80°C | |
| 4 mM ATP | 1 ml | -80°C | |
| 78856 | U2 Assay Buffer | 2 x 10 ml | -80°C |
| 30017 | Anti-His Tb-Labeled Donor | 10 µl | -20°C |
| Dye-Labeled Acceptor | 10 µl | -20°C | |
| 79969 | White, nonbinding, low volume microtiter plate | Room Temp |
* The initial concentration of enzyme is lot-specific and will be indicated on the tube containing the protein.
Ubiquitination is a multistep process that involves E1 (ubiquitin-activating enzymes), E2 (ubiquitin-conjugating enzymes) and E3 (ubiquitin ligase) enzymes. In the first step, an E1-ubiquitin thioester bond is formed between the C-terminal glycine carboxyl group of ubiquitin and the cysteine in the active site of the E1 enzyme in an ATP-dependent reaction. Next, the E1 enzyme transfers the activated ubiquitin to the cysteine residue of the E2 enzyme to form an E2-ubiquitin thioester-linked intermediate by transesterification. Finally, the E2 enzyme transfers ubiquitin to the substrate protein with the help of an E3 ligase. UBA6 (ubiquitin-like modifier activating enzyme 6) is an E1 enzyme that activates ubiquitin but also has the unique feature of being able to act on FAT10 (also known as ubiquitin D or human leukocyte antigen-F adjacent transcript 10). UBA6 is present in most tissues at very low levels, however it is upregulated during dendritic cell maturation and hyperthermic stress. UBA6 can transfer ubiquitin to UbcH5b (also known as Ubiquitin-conjugating Enzyme E2 D2, UBE2D2). UbcH5b is overexpressed in certain cancer types. It ubiquitinates the tumor-suppressor proteins p53 and p62, promoting their degradation. The UbcH5b-p62 complex confers therapeutic resistance in triple negative breast cancer cells. UbcH5b is also involved in endocytosis-mediated degradation of MHC (major histocompatibility complex) class I molecules, and overexpression has been found in inflammatory bowel disease. Inhibitors of ubiquitination of UbcH5b by UBE6 may prove beneficial in cancer and auto-immune disease treatment.
Saville M.K., et al., 2004 J. Biol. Chem.279(40): 42169-81.
Kim J.H., et al., 2015 BMB Rep. 48(1): 25-29.
Truongvan N., et al., 2022 Nature Communications 12:4789.