TR-FRET Assays Simplify and Accelerate Drug Discovery
Introduction
Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) is a powerful technique commonly used to analyze the binding of two interacting molecules. Since most biological responses involve an interaction between at least two partners, TR-FRET is well suited to study a wide range of events, including many that characterize cellular signaling pathways. Possible applications are limitless: interaction between two proteins, between a receptor and its ligand or between an enzyme and its substrate; binding of a drug to its target or binding of a nucleic acid to a protein; measure of post-transcriptional modifications; and more. Proteins of interest can be complexed using a variety of tools such as antibodies, biotin labeling for avidin-biotin interaction, or using click chemistry.

Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) is a powerful technique commonly used to analyze the binding of two interacting molecules. Since most biological responses involve an interaction between at least two partners, TR-FRET is well suited to study a wide range of events, including many that characterize cellular signaling pathways. Possible applications are limitless: interaction between two proteins, between a receptor and its ligand or between an enzyme and its substrate; binding of a drug to its target or binding of a nucleic acid to a protein; measure of post-transcriptional modifications; and more. Proteins of interest can be complexed using a variety of tools such as antibodies, biotin labeling for avidin-biotin interaction, or using click chemistry.
TR-FRET is an ultra-low background technique that allows the measurement of any reaction in which two labeled entities come in proximity. The main drawback of the technique is that it requires two optimized labeled molecules, in addition to having a low dynamic range. However, these drawbacks are offset by several advantages:
- Small volumes
- Homogeneous: no need for washing steps or for physical separation from the unbound entities
- Robust, sensitive signal
- Ultra-low background with high signal-to-noise ratio
- Stable signal: photobleaching is minimized when lanthanide donor fluorophores are used.
The technology is a combination of Time-Resolved Fluorescence (TRF) and Förster’s Resonance Energy Transfer (FRET), a phenomenon in which a light-excited fluorophore can transfer its absorbed energy to a nearby acceptor fluorophore.
FRET
Fluorophores absorb high-energy light and emit light of lower energy than the absorbed light. Fluorescence Resonance Energy Transfer (FRET) is a phenomenon in which two fluorophores emitting at different wavelengths are coupled: the donor fluorophore excited by a high energy source transfers energy (not light) to an acceptor fluorophore. This results in excitation of the acceptor and fluorescence emission at the wavelengths inherent to the properties of the acceptor fluorophore. TR-FRET technology takes advantage of the fact that the transfer of energy between the donor and the acceptor depends on physical proximity (<10 nm) and decreases rapidly with distance. Thus, partner molecules distributed in a solution are sufficiently far apart that FRET does not occur. Upon interaction, the partners complete the FRET pairing as they are now in proximity to each other.
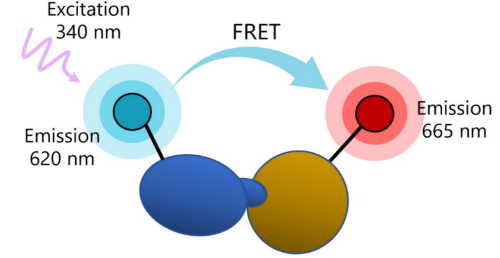
Figure 1: Illustration of FRET principle. In practice, one of the binding partners in the interaction of interest is labeled with a donor fluorophore such as a Europium chelate, whereas the other partner is labeled with an acceptor fluorophore. When direct labeling of the partners is not possible, the molecule of interest is tagged or biotinylated and the pairing is completed using streptavidin-coated donor or acceptor, or anti-tag antibodies.
FRET is applicable to any two interacting molecules, or to reactions in which a new molecular form appears providing that the new molecular form can be distinguished from the initial molecule. Examples include post-transcriptional modifications such as ubiquitination, methylation, or phosphorylation. The molecules under study can be directly labeled with a donor or acceptor fluorophore. Alternatively, a secondary reagent (for example, an antibody) that binds to the molecule of interest can be labeled with one of the fluorophores for indirect detection. This provides great flexibility regarding the design of an assay.
Time-Resolved Fluorescence
FRET has a lower background signal than classic fluorescence methods because the acceptor emission may not share much spectral overlap with the excitation pulse. The donor and the acceptor, on the other hand, must have good spectral overlap (the emission range of the first must overlap with the excitation range of the second), as well as good spectral resolution for a specific signal to be measured. However, it is the Time Resolved Fluorescence (TRF) technology element that allows for the ultra-low background advantage of TR-FRET.
Classic fluorescence intensity uses short-lived fluorophores such as fluorescein, with an emission speed in the order of the nanosecond. Excitation and emission occur at specific wavelengths that can be differentiated by a fluorescence reader. However, excitation and emission happen at the same time. If there is any amount of spectral overlap between excitation and emission, as there usually is, the reader will capture some of the excitation fluorescence, resulting in background signal and low signal-to-noise ratios.
TRF solves this by using long-lived inorganic fluorophores as donors and adding a time delay between excitation and measurement, which means that the excitation signal is gone by the time of the measurement, which considerably decreases background signals (Figure 2).
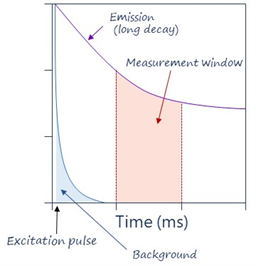
Figure 2: Illustration of TRF principle.
TRF also uses excitation pulses (not continuous excitation), so that a series of measurements are repeated over time. It eliminates the very transient background fluorescence generated from sample components such as buffers, proteins, and chemicals, which hinders classic FRET methods.
Ideal fluorophores have high signal intensity, are highly stable, and offer excellent signal-to-noise ratios. The most commonly used are “Lanthanide probes” which are metal ions referring to elements Cerium to Lutetium in the periodic table, more specifically Europium and Terbium, which fluoresce over milliseconds instead of nanoseconds.
Case Studies
Monitoring PCSK9 binding to LDLR ectodomain.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) directly regulates cholesterol homeostasis and levels of cholesterol in the circulation. It acts by binding to the ectodomain of hepatic low-density lipid receptor family members: low density lipoprotein receptor (LDLR), very low-density lipoprotein receptor (VLDLR), apolipoprotein E receptor (LRP1/APOER) and apolipoprotein E receptor 2 (LRP8/APOER2). The complex is internalized in clathrin-coated endosomes and LDLR is primed for degradation in the lysosomes. As a result of LDLR degradation, LDL-C uptake from the extracellular milieu is decreased, resulting in higher levels in circulation. Gain-of-function mutations in PCSK9 that have been identified in the human population result in higher-than-normal levels of LDL-C and increased risks in cardiovascular diseases, while loss of function mutations confer protection.
PCSK9 inhibitors have emerged as a novel therapeutic class, which reduces LDL-C through increased hepatic clearance and therefore decreases cardiovascular issues, including heart attack and stroke. In particular, neutralizing antibodies such as the FDA-approved Alirocumab (Praluent) and Evolocumab (Repatha) that prevent PCSK9 binding to the LDLR ectodomain have demonstrated clear health benefits.
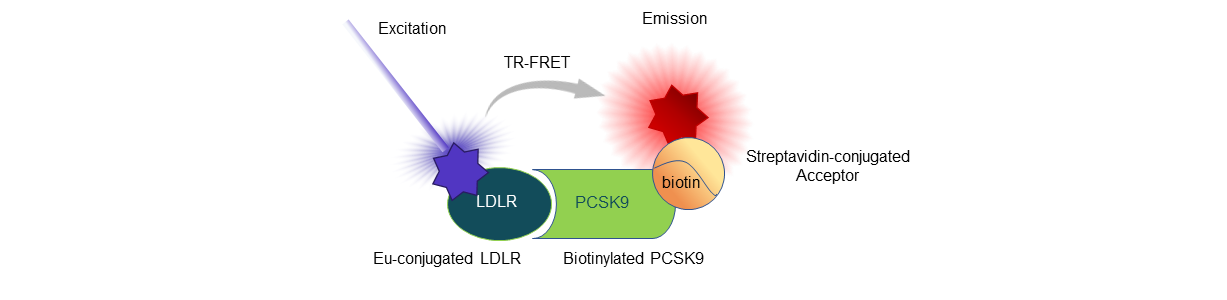
Figure 3: Illustration of the assay principle.
The PCSK9 and PCSK9(D374T)-LDLR TR-FRET Assays are designed for the screening and titration of neutralizing antibodies or other blockers of PCSK9 binding to the LDLR ectodomain. The Eu-conjugated recombinant LDLR ectodomain binds to biotinylated PCSK9 wild-type or mutant. Addition of a streptavidin-conjugated Acceptor, which binds tightly to biotin-PCSK9, completes the TR-FRET pairing. Fluorescence transfer is directly proportional to the amount of binding.
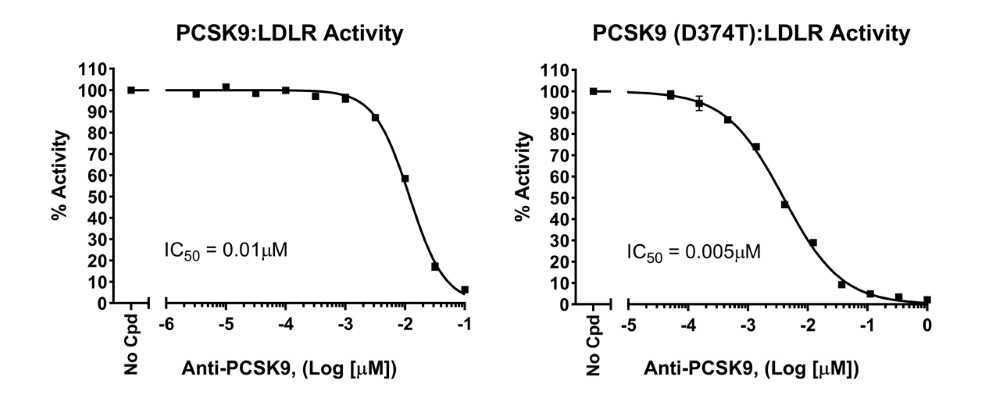
Figure 4: Anti-PCSK9 neutralizing antibody blocks PCSK9 binding to LDLR ectodomain. Eu-conjugated LDLR was pre-incubated with the neutralizing antibody together with the streptavidin-conjugated Acceptor. Biotinylated PCSK9 protein (wild-type or D374T mutant) was added to the reaction and incubated for 2 hours at room temperature before TR-FRET readings. Two sequential measurements were made: Eu-donor emission was measured at 620 nm followed by Acceptor emission at 665 nm. Data analysis was performed using the TR-FRET ratio (665 nm emission/620 nm emission). Results are expressed as percentage of activity, in which TR-FRET signal in the absence of antibody (positive control) was set to 100%. The neutralizing antibody blocked the interaction between PCSK9 and LDLR, causing a decrease in TR-FRET signal.
Measuring ubiquitination
Covalent conjugation of ubiquitin (Ub) to a protein is a common post-translational modification that regulates protein stability, function, and localization. Ubiquitination is the concerted action of three enzymes: a Ub-activating enzyme (E1), a Ub-conjugating enzyme (E2), and a Ub ligase (E3). The specificity and efficiency of ubiquitination are determined by the E3 enzyme, which directs the last step of the conjugation cascade by binding to both an E2-Ub conjugate and a substrate protein, leading to its mono- or poly-ubiquitination.
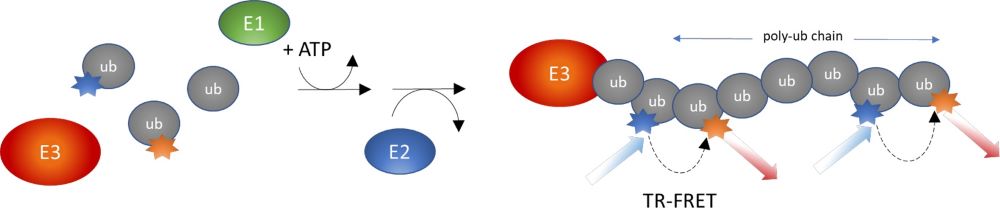
Figure 5: Illustration of the assay principle.
The Intrachain TR-FRET Assay kits were designed to measure the auto-ubiquitination of a specific E3 enzyme in a homogeneous 384-well format. The E3 ligase, such as NEDD4 (neural precursor cell expressed developmentally down-regulated protein 4), MDM2 (mouse double minute 2 homolog), VHL (Von Hippel Lindau), is affinity purified. The assays use a Europium-labeled Ub donor and a Cy5-labeled Ub acceptor to complete the TR-FRET pairing. Since both the donor and acceptor are incorporated into poly-ubiquitin chains forming on the E3 enzyme, the assays measure poly-ubiquitination and not mono-ubiquitination. They are used for high-throughput screening of E3 ligase inhibitors, to perform real-time kinetic analyses, or to accurately determine the IC50 of a compound.
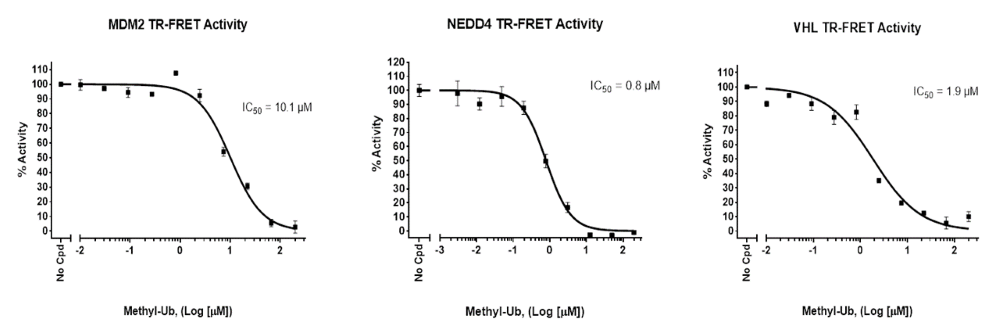
Figure 6: Effect of inhibitor Methyl-Ub on the formation of poly-Ub chains: Increasing concentrations of methyl-ubiquitin were added to a Europium-labeled donor, a Cy5-labeled acceptor, purified E1 and E2 proteins and a purified E3 ligase (NEDD4, MDM2 or VHL) in the presence of ATP, and incubated for 2 hours prior to TR-FRET reading. Two sequential measurements were performed: donor emission was measured at 620 nm followed by dye-acceptor emission at 665 nm.
Conclusion
The growing commercial availability of ready-to-use TR-FRET assay kits has opened the technique to mainstream use. BPS Bioscience offers over 80 assay kits for drug discovery in the TR-FRET format with new products being developed regularly. Optimized, validated, high quality assay kits ensure reliable results, quickly. Alternatively, BPS Bioscience will run the assays for you and test your compounds, saving you time to focus on other critical projects.

