TYK2 JH2 Pseudokinase Domain Inhibitor Screening Assay Kit
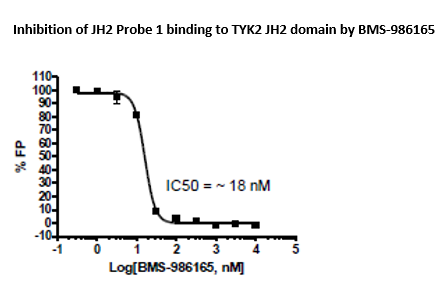
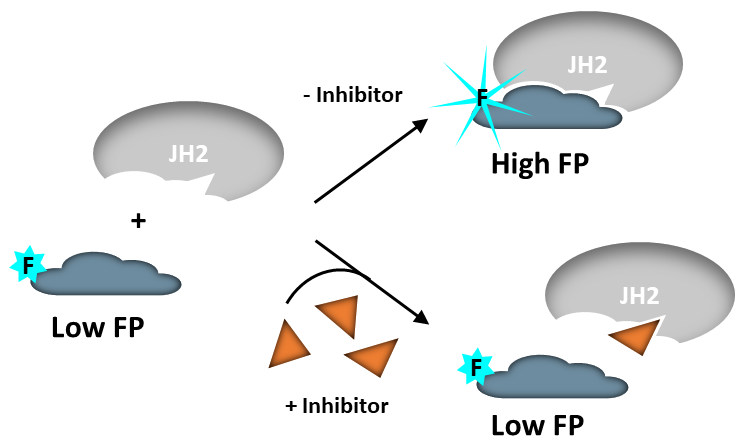
The TYK2 JH2 Pseudokinase Domain Inhibitor Screening Assay Kit is designed for screening and profiling small molecules that displace the fluorescently labeled probe (JH2 probe 1) from the JH2 domain of TYK2. The assay is based on the competition of the test compound with the JH2 probe for binding to purified TYK2 JH2. Using this kit, only one simple step on a microplate is required for screening. The JH2 probe 1 is incubated with a sample containing TYK2 JH2 to produce a change in fluorescent polarization. The FP signal is measured using a fluorescent microplate reader capable of measuring fluorescence polarization.
Adjustable micropipettor and sterile tips
Microplate reader capable of reading fluorescence polarization
| Catalog | Component | Amount | Storage | |
| 100523 | TYK2 (JH2 Domain) | 60 µg | -80°C | Avoid multiple freeze/ thaw cycles! |
| 78103 | JH2 Probe 1, 10 μM (Protect from light) | 10 µl | -80°C | |
| 78106 | JH2 Binding Buffer | 25 ml | -20°C | |
| 384-well black microplate | 1 | Room Temp |
||
Janus kinases (JAKs) are a family of intracellular nonreceptor tyrosine kinases including Jak1, Jak2, Jak3 and TYK2, that has been recognized as an important modulator in inflammatory processes. JAKs contain a catalytically inactive pseudokinase regulatory domain (JH2) as well as an active kinase domain (JH1). Selective inhibition of one specific Jak is a challenging task since the enzymes sharehigh homology in the active site of JH1. Recent reports demonstrate that the pseudokinase domain (JH2) could provide an ideal allosteric site for selective inhibitor development.
Wrobleski, S. T., et al. J. Med. Chem. 2019, 62(20): 8973-8995.