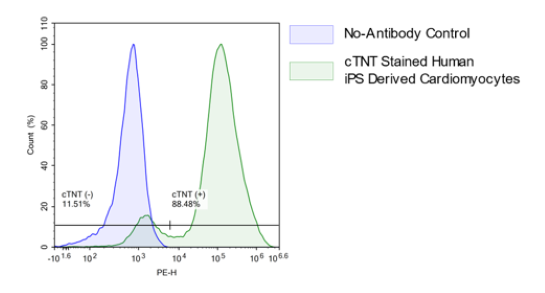
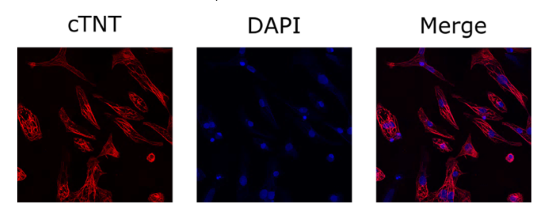
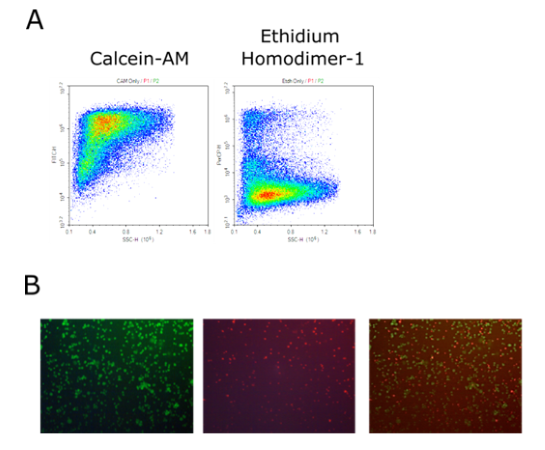
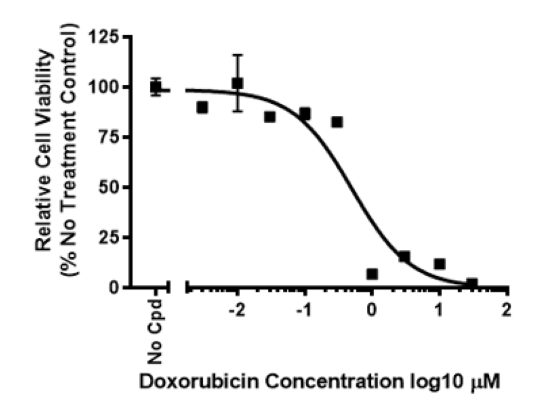
Human iPSC Derived Cardiomyocytes
Human iPSC Derived Cardiomyocytes are non-diseased, non-proliferative human cardiomyocytes differentiated from induced pluripotent stem cells (iPSC) using the small molecule Wnt-modulation strategy described by Lian et al.. The differentiated cells are functional, normal cardiomyocytes useful for in vitro modeling of cardiac biology and drug development studies.
Purchase of this cell line is for research purposes only; commercial use requires a separate license. View the full terms and conditions.
| Name | Ordering Information |
| Thaw Medium Kit C17 | BPS Bioscience #78511 |
| Maintenance Medium C17 | BPS Bioscience #78509 |
| Thiazovivin | BPS Bioscience #78506 |
| DMEM/F12 | ThermoFisher #11330032 |
| Matrigel™ | Corning #354230 |
The cells have been screened to confirm the absence of Mycoplasma species.
The discovery of the Yamanaka factors has enabled the reprogramming of mature human somatic cells to induced pluripotent stem cells with the ability to differentiate along the three germlines lineages involved in human development (endo-, meso- and ectoderm). The impact of this discovery has been most profound in research involving terminally differentiated, non-proliferating cell types which have traditionally been difficult to access.
One of the major causes of the death and burden on the health systems in the developed world are cardiovascular diseases. Human iPSC-derived cardiomyocytes have enhanced our understanding of human cardiac development, congenital heart diseases and mechanisms of drug-induced cardiotoxicity. In addition, the availability of human cardiac muscle cells can transform the drug discovery process. On the one hand, it allows high-throughput phenotypic screening of new drugs targeting cardiac disease. On the other hand, it allows cardiotoxicity studies to be performed very early on in the drug discovery without using more expensive, and ultimately less clinically relevant mouse models. These two aspects combined are expected to decrease the cost of drug development, since cardiac toxicity is a major cause of attrition in drug development pipelines. The ability to use a clinically relevant, amenable system to deepen our understanding of cardiac cell biology and drug responses can result in major benefits for the ageing population and economy of developed countries.
Takahashi K., et al., 2007, Cell 131: 861-872.
Yamanaka S., et al., 2012, Cell Stem Cell 10: 678-684.
Lian X., et al., 2012, PNAS 109(27): E1848-E1857.
Musunuru K., et al., 2018 Circulation: Genomic and Precision Medicine 11: e000043.
Mordwinkin., et al., 2013, Journal of Cardiovascular Translational Research 6: 22-30