PARP2 Olaparib Competitive Inhibitor Assay Kit
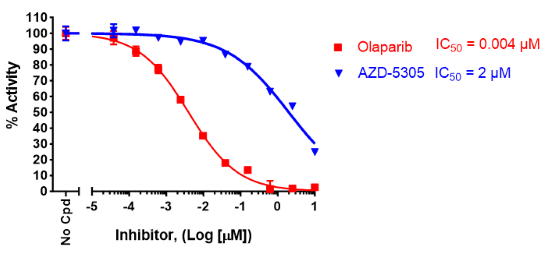
The PARP2 Olaparib Competitive Inhibitor Assay Kit is a competitive FP (fluorescent polarization) assay designed to measure the formation of a complex between PARP2 (poly(ADP-ribose) polymerase 2) and a fluorescent probe that contains the PARP2 inhibitor Olaparib. When the probe is bound to PARP1, FP is high. In the presence of a test compound able to bind to the same site in PARP2 as Olaparib, the Olaparib-containing fluorescent probe is displaced from PARP1 and remains in solution, resulting in low FP. The PARP2 Olaparib Competitive Inhibitor Assay Kit comes in a convenient 96-well format, with purified PARP2 enzyme (amino acids 2-583), Olaparib-containing fluorescent-labeled probe, and assay buffer for 100 enzyme reactions.
Note: This kit is not appropriate for inhibitors expected to bind to PARP2 at different sites from Olaparib.
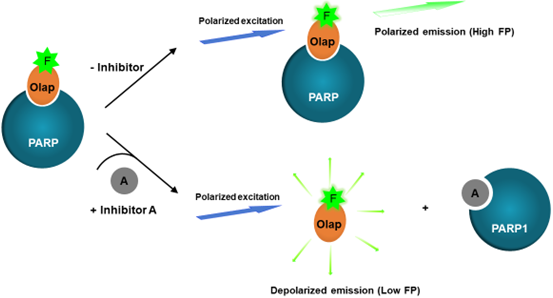
Figure. 1: PARP2 Olaparib Competitive Inhibitor Assay Kit mechanism.
PARP2 binds to the Olaparib-containing fluorescent probe, forming a complex. This complex, when subjected to polarized excitation light, emits highly polarized light due to its restricted movement in solution. In the presence of a test compound (A), PARP2 may form a complex with either the test compound, if the compound has the same binding site on PARP2 as Olaparib, or with the Olaparib-containing fluorescent probe. If the test compound binds to PARP2, the Olaparib-containing fluorescent probe remains in solution and rotates freely what is manifested by low FP. The decrease in FP value is proportional to the competitive binding of the test compound to PARP2.
This assay requires a fluorescent microplate reader capable of measuring fluorescence polarization (FP) to read the FP signal. For more information FP technology, visit our Tech Note: FP, assay principles and applications.
- Adjustable micropipettor and sterile tips
- Rotating or rocker platform
- Fluorescent microplate reader capable of measuring fluorescence polarization (λexc=485/20 nm and detection at λem=528/20 nm)
| Catalog # | Name | Amount | Storage |
| 80502 | PARP2, GST-Tag* | 8 µg | -80°C |
| PARPi-FL (10 µM) | 5 µl | -80°C | |
| 5x PARPtrap™ Assay Buffer 2 | 2 x 1 ml | -80°C | |
| 79685 | 96-well black microplate | 1 | Room Temp |
* The initial concentration of enzyme is lot-specific and will be indicated on the tube containing the protein.
PARP2, also known as poly-(ADP-ribose) polymerase 2 or NAD+ ADP-ribosyltransferase 2, is part of the PARP family. ADP ribosylation, which is the addition of an ADP-ribose to a protein, is a reversible post-translational modification of proteins mostly involved in the DNA Damage Response (DDR) pathway. Poly-ADP-ribosylation (termed PARylation) is the addition of linear or branched chains of ADP-ribose. PARP2 participates in DNA repair (only 10% of total PARP activity is due to PARP2), but also in oxidative stress and mitochondrial fragmentation. Dysfunction of the DDR and oxidative stress pathways can lead to oncogenesis. Genetic ablation of PARP2 has indicated roles of PARP2 in adipogenesis, spermatogenesis and thymocyte survival. It is also a co-factor of nuclear receptors like ER (estrogen receptor) and PPAR (peroxisome proliferator-activated receptors). PARP2 is overexpressed in prostate cancer and may contribute to the disease through the FOXA1 (forkhead box protein A1)/AR pathway. PARP inhibitors have been used in cancer treatment with success, with the clinically approved inhibitors targeting both PARP1 and PARP2. Further understanding of the molecular pathways involving PARP2, and its contribution to disease, will continue to pave the way for new therapies for PARP2-linked diseases.
Gui B., et al., 2019 Proc Natl Acad Sci USA 116 (29): 14573-14582.