OX40 Signaling and Its Implications for Immunotherapy
Introduction
![]() Immune checkpoint OX40, also known as CD134 or TNFRSF4, is a co-stimulatory molecule belonging to the tumor necrosis factor receptor (TNFR) superfamily. Its significance in regulating immune responses has garnered substantial attention in the field of immunotherapy. OX40 signaling plays a crucial role in modulating T cell activation, differentiation, and survival, thereby influencing the outcome of immune responses against pathogens and tumors. This technical note aims to elucidate the mechanisms underlying OX40 signaling and its implications for immunotherapy.
Immune checkpoint OX40, also known as CD134 or TNFRSF4, is a co-stimulatory molecule belonging to the tumor necrosis factor receptor (TNFR) superfamily. Its significance in regulating immune responses has garnered substantial attention in the field of immunotherapy. OX40 signaling plays a crucial role in modulating T cell activation, differentiation, and survival, thereby influencing the outcome of immune responses against pathogens and tumors. This technical note aims to elucidate the mechanisms underlying OX40 signaling and its implications for immunotherapy.
OX40 Signaling Pathway
OX40 is mainly expressed by activated CD4+ and CD8+ T cells and is transiently induced upon TCR stimulation. The activation of OX40 occurs upon binding to its ligand, OX40L, also known as CD252, TNFSF4 or gp34, which is primarily expressed on antigen-presenting cells (APCs) such as dendritic cells, macrophages, and B cells. OX40L trimers induce OX40 receptor clustering, which initiates a signaling cascade that involves various intracellular adaptors and kinases. Upon ligand binding, OX40 recruits TNF receptor-associated factors (TRAFs), particularly TRAF2 and TRAF5, leading to the activation of downstream signaling pathways, including NF-κB, MAPK, and PI3K/Akt. TRAFs interact directly with receptor clusters and are not mediated by phosphorylation like many other cell surface signaling receptors. These pathways collectively promote T cell proliferation, survival, and cytokine production, thereby enhancing immune responses. OX40 also acts as a costimulatory receptor in the context of TCR activation. When both receptors are engaged, OX40 signaling enhances activation of the Akt pathway, further amplifying growth and survival signals.
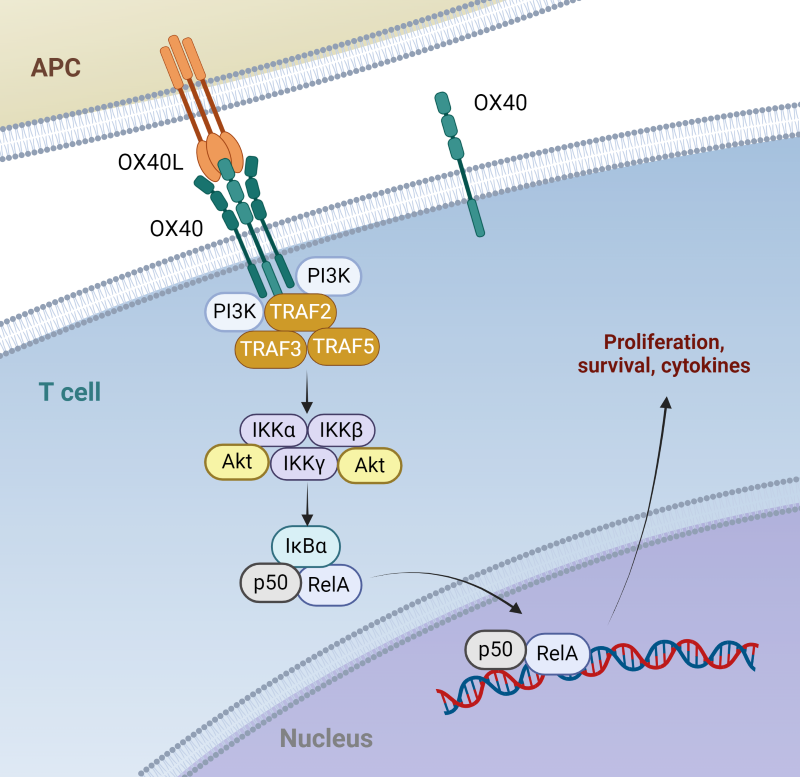
Figure 1. OX40:OX40L signaling pathway independent of TCR signaling. Adapted from Croft, M. 2010 (1).
OX40 Drives a Diversity of Functions Across T Cell Subsets
OX40 is expressed on a variety of T helper subsets, particularly, Th2, Th1, Th17, Tfh, as well as regulatory T cells (Tregs) (2). Specifically, OX40–OX40L signals can enhance the Th1-mediated immune response, promote the generation and maintenance of Th2 responses, augment Tfh development, influence IL-17 production from Th17 cells, and antagonize Treg generation and Treg-mediated suppression. In CD8+ cytotoxic T cells, OX40 engagement has been found to be critical for survival and expansion of primed CD8+ T cells, as well as their responses to antigen stimulation.
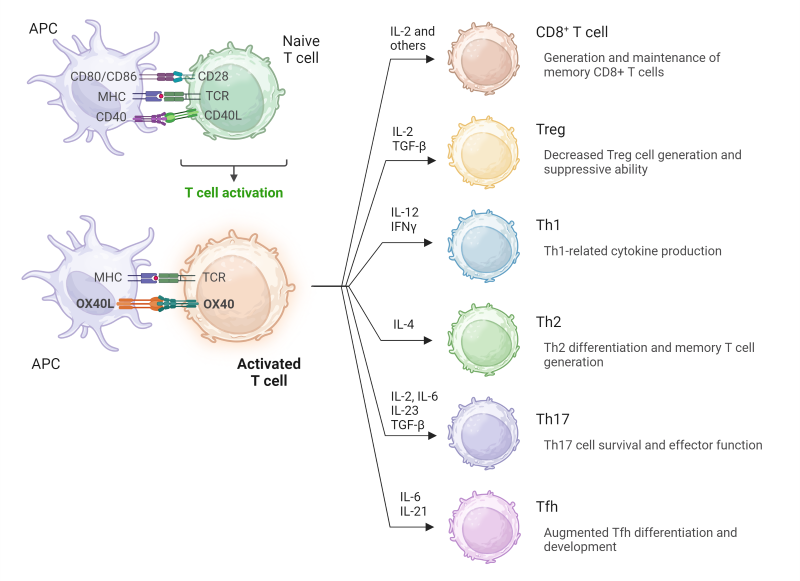
Figure 2. Summary of OX40 stimulation across T cell subsets. Adapted from Fu, Y. et.al. 2020 (2).
Implications for Immunotherapy
The dysregulation of OX40 signaling has been implicated in various immune-related disorders, including autoimmune diseases and cancer (2). In cancer, tumor cells often exploit inhibitory pathways to evade immune surveillance, particularly systems that induce T cell exhaustion. Immunotherapeutic strategies targeting OX40 aim to reverse this immune suppression and potentiate anti-tumor immunity, reinvigorating T cells in tumor compartments. Preclinical studies have demonstrated the efficacy of OX40 agonists in enhancing T cell responses against tumors by promoting effector T cell activation and memory formation. Furthermore, augmenting OX40 activity may be useful as a therapeutic adjuvant in vaccinations or for enhancing immunity against pathogens, such as viruses, fungi, or helminths.
Several OX40 agonists have entered clinical trials for the treatment of various cancers. These agonists include monoclonal antibodies targeting OX40, soluble OX40L fusion proteins, and OX40 agonist antibodies conjugated to immunomodulatory agents or cytotoxic payloads. Early-phase clinical trials have shown promising results, with some patients achieving durable responses. However, challenges such as off-target effects and limited efficacy in certain tumor types remain to be addressed.
Given the complexity of tumor immune evasion mechanisms, combination therapies involving OX40 agonists are being explored to enhance their efficacy. Combinations with other immunomodulatory agents, such as checkpoint inhibitors targeting PD-1/PD-L1 or CTLA-4, aim to overcome multiple layers of immune suppression within the tumor microenvironment. Additionally, synergistic effects have been observed with conventional cancer therapies, such as chemotherapy and radiation therapy, which can augment the immunogenicity of tumors and sensitize them to immune-mediated destruction.
On the other hand, blocking OX40 signaling may improve autoimmune disease outcomes where overactive immune cells are driving the pathology. A handful of clinical trials targeting OX40 or OX40L for autoimmunity are under way. OX40 has been implicated in a variety of autoimmune disorders, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), inflammatory bowel disease (IBD), autoimmune experimental uveitis (AEU), type 1 diabetes mellitus, and multiple sclerosis (MS). Indeed, OX40 expression is upregulated at the sites of autoimmunity and correlates with disease severity. Thus, targeting OX40 or OX40L with blocking antibodies may be an ideal therapeutic approach.
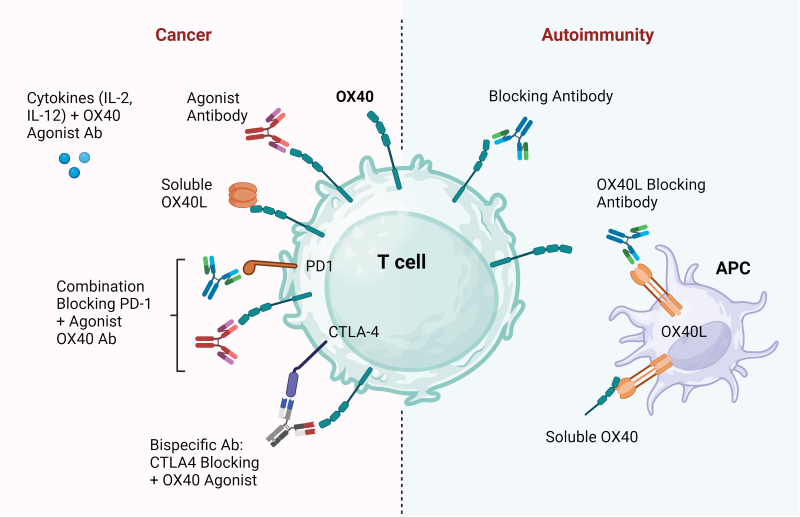
Figure 3. Therapeutic strategies to target OX40:OX40L in cancer and autoimmunity. In cancer, the therapies would promote T cell activation, while in autoimmunity, therapies would aim to suppress T cell function.
Tools for OX40:OX40L Drug Discovery
BPS Bioscience delivers an array of tools to expedite drug discovery, including proteins, blocking antibodies, biochemical assays, and reporter cell lines.
For compound library or antibody screening and profiling, the OX40[Biotinylated]:OX40L Inhibitor Screening Assay Kit (#72045) provides a convenient, all-in-one solution with a robust chemiluminescent readout.
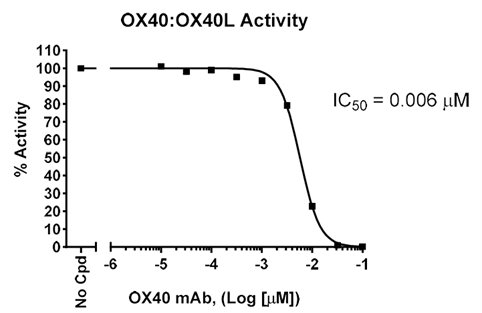
Figure 4. Inhibition of binding of OX40:OX40L by an anti-OX40 antibody. A plate was coated with OX40L, followed by incubation with OX40 in the presence of increasing concentrations of Anti-OX40 Antibody. Luminescence was measured using a Bio-Tek microplate reader.
Our OX40 blocking antibodies are suitable for controls in your discovery assays.
Anti-OX40 Competitive Antibody
BPS Bioscience reporter cell lines provide a biologically relevant context for screening and profiling potential therapeutics. The OX40/NF-κB Luciferase Reporter HEK293 Cell Line (#60482) provides a clear signal against a low background control, enabling determination of IC50 of blocking antibodies. Using our ONE-Step™ Luciferase Assay System, researchers can run high-throughput assays to pinpoint the best candidates to advance through the pipeline.
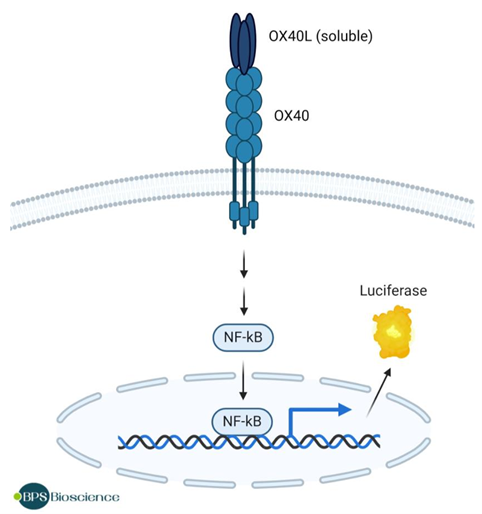
Figure 5. Illustration of the mechanism of action of the OX40/NF-κB Luciferase Reporter HEK293 Cell Line.
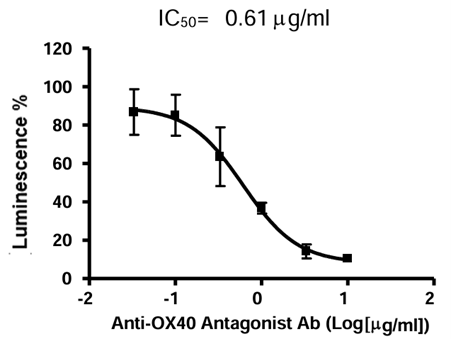
Figure 6. Dose-response curve of OX40/NF-κB Luciferase Reporter HEK293 Cell Line to Anti-OX40 Antagonist Antibody in the presence of OX40L. Cells were incubated with increasing concentrations of Anti-OX40 Antagonist Antibody and 400 ng/ml of OX40L for 6 hours. Luciferase activity was measured using ONE-Step™ Luciferase Assay System. The results are shown as fold induction of luciferase reporter expression in relation to the activity of cells in the absence of OX40L.
References
1. Croft, M. 2010. Annu Rev Immunol. 28:57-78. Pubmed
2. Fu, Y., et.al. 2020. Act Pharm Sin B. 10:414-433. Pubmed
3. Figures 1, 2, and 3 were created with BioRender.com.
│Related Products
- OX40/NF-κB Luciferase Reporter HEK293 Cell Line
- OX40 Recombinant Proteins
- OX40L Recombinant Proteins
- OX40 Antibodies
- OX40:OX40L Inhibitor Screening Assay Kit
│Related Services
- OX40 Screening Services
- Cell Signaling Pathway Screening Services
- Immunotherapy Biochemical Screening Services

