Kill Curve Protocol
A kill curve allows for determination of the optimal concentration of selection antibiotic to use for selection of an engineered cell line having integrated a construct of interest.
Background
A kill-curve is a dose-response experiment in which cells are cultivated in the presence of increasing concentrations of a specific antibiotic for a period of one week to ten days. Slow-growing cells may need to stay under selection for up to 15 days depending on their growth rate. The minimum antibiotic concentration that is both required and sufficient to kill all the cells is then used for antibiotic selection, and for maintaining the stability of the genetically modified cell line.
Protocol
- Plate the cells in a 24-well plate in the relevant complete growth medium, at a density (cell number/well) that allows cells to reach approximately 30-50% confluency a day later.
- The day after plating, add increasing concentrations of the antibiotic of interest to the growth medium. Include a medium-only (no antibiotic) control.
- Replace the cell culture medium, maintaining the antibiotic concentration, every 3-4 days for up to 10 days. Keep in mind that some antibiotics may have a short half-life due to lower stability in solution.
- Examine the cells under a microscope every day for signs of cell death.
- On day 10, determine cell viability in each well using Trypan Blue staining or by accurate cell counter.
- Make note of the antibiotic concentration that is necessary and sufficient to kill all cells after 10 days.
Tips and Tricks
- The medium exchange schedule should be adjusted according to cell type and antibiotic. Some antibiotics are less stable than others in solution, please refer to the information provided by the manufacturer.
- It is recommended to perform the dose-response in duplicate.
- Example of experimental set up:
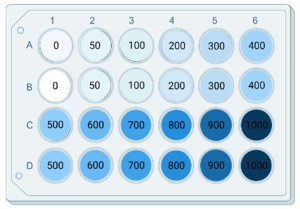
- The useful range of concentration depends on the antibiotic.
Examples:
G418 0.1 to 2.0 mg/ml
Hygromycin 100 to 500 µg/ml
Puromycin 0.25 to 10 µg/ml - Genetic engineering being done sequentially, if using a parental cell line containing a genetic modification and growing under selection pressure, one should perform the dose response of the second antibiotic using cells cultured in growth medium that contains the first antibiotic. The useful concentration of a third antibiotic similarly would be tested in the presence of the first two.
Contact Technical Support
│ Related Products
- Cell Lines
- iPSC-Derived Cells
- Cell-Based Assays and Expression Kits
- Cell Media & Luciferase Reagents

