iPSC-Derived Cells
Induced pluripotent stem cells (iPSC), pioneered by Nobel Prize winner Shinya Yamanaka, are generated by converting mature human somatic cells via expression of four critical transcription factors known as Yamanaka factors: Myc, Sox2, Klf4, and Oct3/4. iPSCs are an important tool for research because they are capable of differentiating into various cell types, including those of different tissues and organs. They can be used to study disease mechanisms, drug development, and regenerative medicine, among other applications. Additionally, iPSCs can be generated from patient cells, allowing for the creation of disease-specific models that can be used to better understand the underlying pathology and develop targeted treatments. BPS Bioscience enables research applications by providing iPSCs, iPSC-derived cells, and engineered reporter iPSC-derived cells.
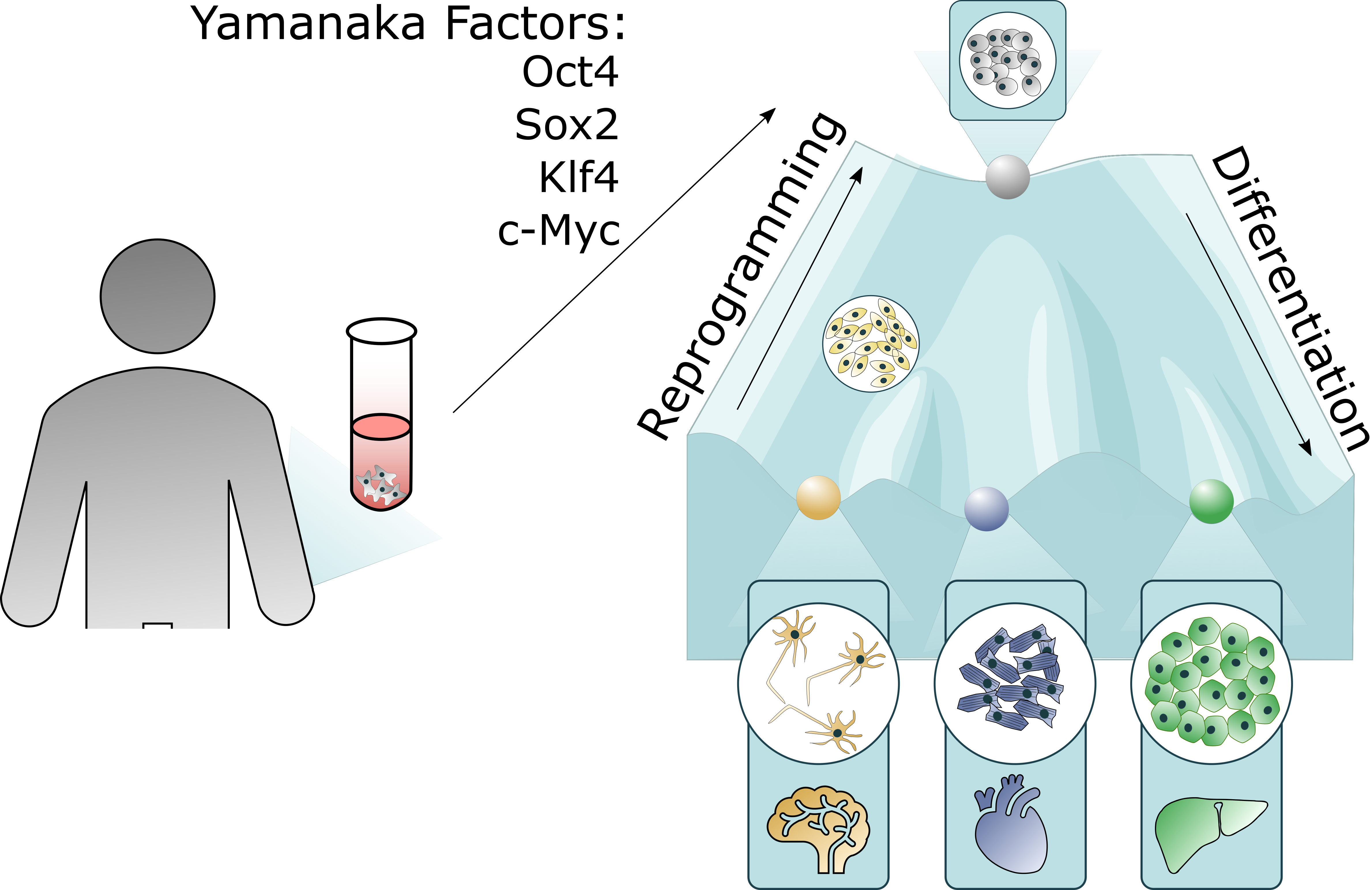
iPSC-Derived Cells Enable Drug Discovery & Cell Therapy Research
Benefits
- Improved model fidelity over cell lines and biochemical assays
- Improved throughput and cost over animal models
High-Throughput Reporters

Use luciferase or eGFP to quantitate transcription factor activation or use constitutive expression to monitor cells in co-culture assays.
Toxicity/Viability Screening

Use iPSCs to assess toxicity of compounds in your differentiated cell types of interest.
Stem Cell Therapy Models

Use iPSCs or derived cells to assess uptake and incorporation into tissues with in vivo imaging.
High-Throughput Reporters

Use luciferase or eGFP to quantitate transcription factor activation or use constitutive expression to monitor cells in co-culture assays.
Toxicity/Viability Screening

Use iPSCs to assess toxicity of compounds in your differentiated cell types of interest.
Stem Cell Therapy Models

Use iPSCs or derived cells to assess uptake and incorporation into tissues with in vivo imaging.
Available Products & Services
Human iPSC Derived Cardiomyocytes
Human iPSC Derived Cardiomyocytes are non-diseased, non-proliferative human cardiomyocytes differentiated from induced pluripotent stem cells (iPSC) using the small molecule Wnt-modulation strategy described by Lian et al. The differentiated cells are functional, normal cardiomyocytes useful for in vitro modeling of cardiac biology and drug development studies.
View Products
StemBright™ Reporter iPSCs
StemBright™ Reporter iPSCs and iPSC-derived cells (neural progenitors or cardiomyocytes) are engineered to express a conditional reporter gene that will respond to the activation of a transcription factor within a cell signaling pathway of interest, or to constitutively express luciferase or eGFP for cell tracking. For example, Wnt signaling is involved in the cell cycle re-entry of iPSC-derived cardiomyocytes. The TCF/LEF (Wnt) Luciferase Reporter iPS Cell pool responds to Wnt pathway activators with a quantitative, dose-dependent increase in luciferase activity. These pluripotent reporter cells can also be used to generate differentiated reporter cells.
View ProductsCas9-Expressing iPSCs
PBMC-derived human iPS cells were engineered to express Cas9 (Streptococcus pyogenes CRISPR associated protein 9) either constitutively or upon doxycyclin induction (tet-on). These cells can be transfected or transduced with a cDNA encoding single-guide RNA(s) targeting specific gene(s) of interest to generate knockout, mutated, or knockin cells. The inducible cells allow temporal control of Cas9 expression, which limits the occurrence of off-target modifications.
View ProductsKnockout iPSCs

Eliminating the expression of a cellular protein can provide valuable research insights. Using CRISPR/Cas9 technology, we can effectively knockout target proteins to change cellular properties as desired. For example, our B2M Knockout iPS Cell Line has the full functional capacity of iPSCs, but has very low immunogenicity, providing a useful tool for allogeneic cell therapy research.
View ProductsEngineering, Differentiation, and Screening Services

Accelerate your research by letting us design your engineered iPSC of interest, differentiate large numbers of cells, or screen your compounds of interest in our iPSC or differentiated cells, so you can focus on other facets of your research. We will provide a comprehensive report with both raw and analyzed data, graphs, detailed protocols, and other important information. Restrictions apply.
Request Service
Human iPSC Derived Cardiomyocytes
Human iPSC Derived Cardiomyocytes are non-diseased, non-proliferative human cardiomyocytes differentiated from induced pluripotent stem cells (iPSC) using the small molecule Wnt-modulation strategy described by Lian et al. The differentiated cells are functional, normal cardiomyocytes useful for in vitro modeling of cardiac biology and drug development studies.
View Products
StemBright™ Reporter iPSCs

StemBright™ Reporter iPSCs and iPSC-derived cells (neural progenitors or cardiomyocytes) are engineered to express a conditional reporter gene that will respond to the activation of a transcription factor within a cell signaling pathway of interest, or to constitutively express luciferase or eGFP for cell tracking. For example, Wnt signaling is involved in the cell cycle re-entry of iPSC-derived cardiomyocytes. The TCF/LEF (Wnt) Luciferase Reporter iPS Cell pool responds to Wnt pathway activators with a quantitative, dose-dependent increase in luciferase activity. These pluripotent reporter cells can also be used to generate differentiated reporter cells.
View ProductsCas9-Expressing iPSCs
StemBright™ Reporter iPSCs and iPSC-derived cells (neural progenitors or cardiomyocytes) are engineered to express a conditional reporter gene that will respond to the activation of a transcription factor within a cell signaling pathway of interest, or to constitutively express luciferase or eGFP for cell tracking. For example, Wnt signaling is involved in the cell cycle re-entry of iPSC-derived cardiomyocytes. The TCF/LEF (Wnt) Luciferase Reporter iPS Cell pool responds to Wnt pathway activators with a quantitative, dose-dependent increase in luciferase activity. These pluripotent reporter cells can also be used to generate differentiated reporter cells.
View ProductsKnockout iPSCs

Eliminating the expression of a cellular protein can provide valuable research insights. Using CRISPR/Cas9 technology, we can effectively knockout target proteins to change cellular properties as desired. For example, our B2M Knockout iPS Cell Line has the full functional capacity of iPSCs, but has very low immunogenicity, providing a useful tool for allogeneic cell therapy research.
View ProductsEngineering, Differentiation, and Screening Services

PBMC-derived human iPS cells were engineered to express Cas9 (Streptococcus pyogenes CRISPR associated protein 9) either constitutively or upon doxycyclin induction (tet-on). These cells can be transfected or transduced with a cDNA encoding single-guide RNA(s) targeting specific gene(s) of interest to generate knockout, mutated, or knockin cells. The inducible cells allow temporal control of Ca9 expression, which limits the occurance of off-target modifications.
Request ServiceiPS Cells License Disclosure
The iPSC technology is protected by several patents, including US patent Nos. 8048999, 8058065, 8129187, 8278104, 8530238, 8900871, 9404124, 9499797, 10519425, and patent pending, for which iPS Academia Japan, Inc. has been granted license rights with a sub-licensable right. The purchase of this cell line grants you a 10-year license to use this cell line in your immediate laboratory for internal research purposes. Commercial use of this cell line is not allowed. Commercial use requires the appropriate license from iPS Academia Japan, Inc. This cell line is for research use only, not for therapeutic or prophylactic use in humans or animals. Use in humans is strictly prohibited.
This license does not permit you to share, distribute, sell, sublicense, or otherwise make the cell line available for use to other laboratories, departments, research institutions, hospitals, universities, or biotech companies. The license does not permit modification of the cell line in any way. Modification of this cell line or transfer to another facility of the cells requires a separate license or additional fees; contact [email protected] for details. Publications using this cell line should reference BPS Bioscience, Inc., San Diego.
Inappropriate use or distribution of this cell line will result in revocation of the license and result in an immediate cease of sales and distribution of BPS products to your laboratory. BPS does not warrant the suitability of the cell line for any particular use and does not accept any liability in connection with the handling or use of the cell line.
Quote Request
Inquiries
│Related Products
- Human iPSC Derived Cardiomyocytes
- StemBright™ iPSC Reporter Cells
- Knockout iPS Cell Lines
- Cell Lines & Primary Cells
- Cell Media & Luciferase Reagents

