HSP90α, His-tag Recombinant
Catalog #
50290
$435
*
●
●
Purchase
Description
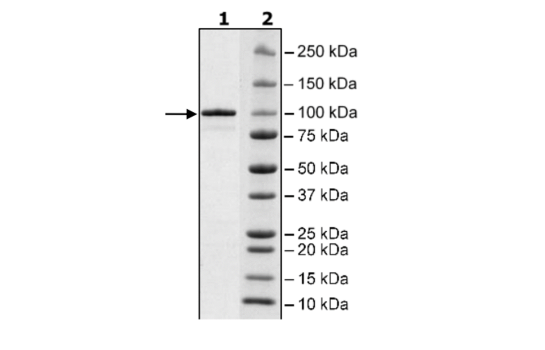
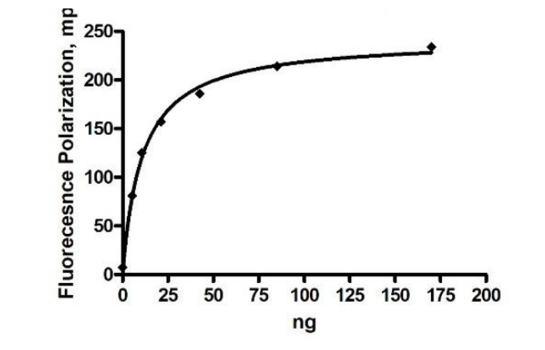
Recombinant human HSP90α (heat shock protein 90 alpha family class A member 1), full length encompassing amino acids 1-732 (end). The construct contains a C-terminal His-tag (6xHis). The protein was affinity purified.
This product has been cited 8 times.
●
Synonyms
HSP90a, Heat Shock Protein 90a, HSP90alpha, HSP90α, EL52, HSPN, LAP2, Heat shock 86 kDa, HSP86, HSPC1, HSPCA, Hsp89, Hsp90, Lipopolysaccharide-associated protein, LPS-associated protein 2, LAP-2, HSP89A, HSP90A, HSP90N, Hsp103, HSPCAL1, HSPCAL4, HEL-S-65p
●
Product Data Gallery
Product Info
Storage and Usage
Citations8
Species
Human
Construct
HSP90α (1-732(end)-His)
Host Species/Expression System
E. coli
Purity
≥90%
Format
Aqueous buffer solution
Formulation
45 mM Tris-HCl, pH 8.0, 124 mM NaCl, 2.4 mM KCl, 10% glycerol, 3 mM DTT, variable imidazole
MW
86 kDa
Amino Acids
1-732(end)
Genbank #
NM_005348
UniProt #
P07900
Background
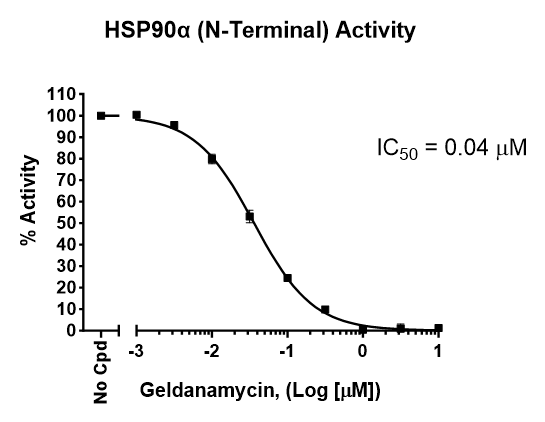
Hsp90 (90 kDa heat shock protein) is a molecular chaperone that aids protein folding and quality control for a large number of client proteins. Functional Hsp90 operates as dimer and has intrinsic ATPase activity. The Hsp90 dimer acts in concert with other chaperones (e.g. Hsp70) and is regulated by a number of co-chaperones/accessory proteins (e.g. Hop, cdc37). Hsp90 has been shown to interact with > 100 proteins and some notable clients include kinases (e.g. Raf-1), nuclear hormone receptors (e.g. estrogen receptors), transcription factors (e.g. p53), GPCRs (e.g. CB2 receptors) and ion channels (e.g. CFTR). In humans, the
Hsp90beta isoform is constitutively expressed whereas the Hsp90alpha isoforms is expressed under stress conditions. Hsp90 plays an important role in some tumor cell types by stabilising mutated oncogenic
proteins.
References
1. Wayne N. and Bolon D.N. J Biol Chem. 2007 Nov 30;282(48):35386-95.
2. Pearl L.H., et al. Biochem J. 2008 Mar 15;410(3):439-53.