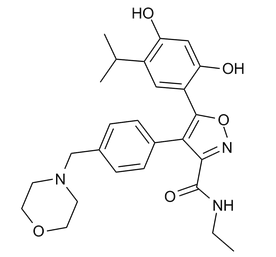
NVP-AUY922
Catalog #
27026
$150
*
●
●
Purchase
Description
NVP-AUY922 potently inhibits HSP90 with IC50 values of 7.8 nM and 21 nM, and Ki values of 9 nM and 8.2 nM for HSP90α and HSP90β, respectively. NVP-AUY922 shows a very high binding affinity to HSP90β with a Kd of 1.7 nM. NVP-AUY922 inhibits the proliferation of human tumor cells in vitro with GI50 values of approximately 2 to 40 nM, inducing G1-G2 arrest and apoptosis. Human endothelial cells are very sensitive to NVP-AUY922 with GI50s of 2.5-3.9 nM. NVP-AUY922 also exhibits potent antitumor efficacy in human tumor xenografts including BT474 breast, A2780 ovarian, U87MG glioblastoma, PC3 prostate, and WM266.4 melanoma.
●
Synonyms
AUY922, VER-52296
●
Product Data Gallery
Product Info
Storage and Usage
Citations
Purity
≥99%
Format
White to off-white solid.
Formula
C26H31N3O5
MW
465.5 Da
Solubility
Soluble in DMSO at 100 mg/ml; soluble in ethanol at 100 mg/ml; very poorly soluble in water; maximum solubility in plain water is estimated to be about 25-50 µM; buffers, serum, or other additives may increase or decrease the aqueous solubility.
Biological Activity
NVP-AUY922 potently inhibits the proliferation of gastric cancer cell lines with with GI50 values of approximately 2 to 40 nmol/L and significantly induces the degradation of growth factor receptors and other client proteins. NVP-AUY922 significantly suppressed the activity of AKT and ERK and potently induced antiproliferation in ESCC cells.
CAS Registry #
747412-49-3
Background
Heat shock protein 90 (HSP90) is a molecular chaperone essential for the stability of key regulators of cell growth and survival. HSP90 is involved in protein folding and functions as a chaperone for numerous client proteins, many of which are important in cancer pathogenesis. Hsp90 enables cancer cell survival by maintaining the function of their mutated client proteins including the KIT and PDGFRA proteins. AUY922 inhibits Hsp90, resulting in proteasomal degradation of oncogenic client proteins, inhibition of cell proliferation, and elevation of HSP72 levels in a wide range of human tumor cell lines.
References
Eccles, S.A., et al. Cancer Res. 68: 2850-2860 (2008).