Caspase3, His-Tag Recombinant
Only %1 left
Catalog #
80500
As low as
$285
*
●
●
Purchase
Description
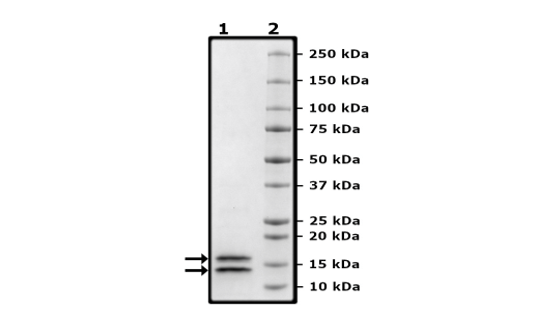
Recombinant human Caspase3 (CASP3), full length, encompassing amino acids 1-277(end). This construct contains a C-terminal His tag (6xHis). Procaspase-3 is self-cleaved into two subunits, MW = 17 kDa and 13 kDa, to form the active heterotetrameric complex. The recombinant protein was affinity purified.
This product has been cited 1 time.
●
Synonyms
caspase-3, CASP3, caspase 3, CPP32, apopain, Yama, Caspase
●
Product Data Gallery
Product Info
Storage and Usage
Citations1
Species
Human
Construct
Caspase3 (1-277(end)-His)
Host Species/Expression System
E. coli
Purity
≥90%
Format
Aqueous buffer solution
Formulation
40 mM Tris-HCl, pH 8.0, 110 mM NaCl, 2.2 mM KCl, 0.04% Tween-20, 20% glycerol, and 3 mM DTT
MW
Small active subunit: 13 kDa; Large active subunit: 17 kDa
Amino Acids
1 - 277 (end)
Specific Activity
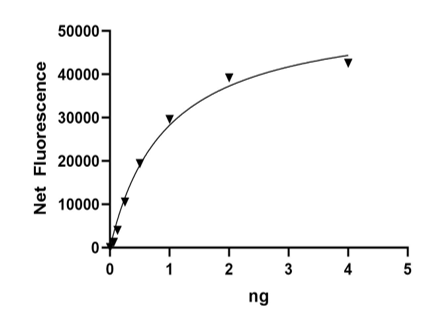
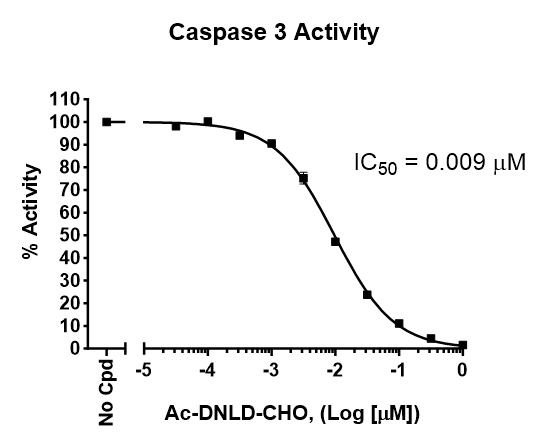
≥6784 pmol/min/µg
Genbank #
NM_004346
UniProt #
P42574
Background
Caspase-3 is a member of the caspase (cysteine aspartate protease) family of proteins, and has been shown to be an executioner protein of apoptosis.
References
1. Lee, D., et al., J Biol Chem. 2000 May 26;275(21):16007-14
2. Suzuki, Y., et al., Proc Natl Acad Sci USA. 2001 Jul 17;98(15):866-7.
2. Suzuki, Y., et al., Proc Natl Acad Sci USA. 2001 Jul 17;98(15):866-7.