Sortase A Assay Kit
The Sortase A Assay Kit is a fluorogenic assay kit designed to measure Sortase A proteolytic activity for screening and profiling applications.
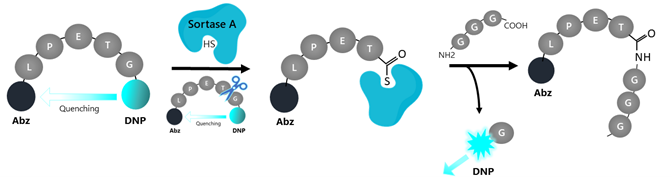
Figure 1: Illustration of the assay principle.
The fluorescence emitted by donor DNP is quenched due to the proximity of the Abz acceptor in the intact peptide. The protease cleaves the peptide between T (threonine) and G (glycine) and forms an intermediate complex between the cysteine residue within the active site and the C-terminus of the peptide substrate. The cleaved substrate is then covalently bounded to the triglycine nucleophile. The glycine-DNP fragment is released, freeing the emission of fluorescence by DNP.
Need us to run inhibitor screens or profile your compounds against Sortase A? Check out our Protease Screening Services.
- Microplate reader capable of reading fluorescence
- Adjustable micropipettor and sterile tips
- 30°C incubator
| Catalog # | Name | Amount | Storage | |
| 71086 | Sortase A, His-Tag* | 12.5 μg | -80°C | Avoid multiple freeze/ thaw cycles! |
| 79938 | 2x Sortase assay buffer | 2.5 ml | -20°C | |
| 79939 | Triglycine | 250 μl | -20°C | |
| 79940 | Abz/Dnp substrate | 250 μl | -20°C | |
| 79685 | Low binding, black NUNC 96-well plate | 1 | Room Temp | |
*The concentration of Sortase A is lot-specific and will be indicated on the tube containing the enzyme.
Staphylococcal Sortase A is a bacterial transpeptidase that covalently attaches proteins to the bacterial cell wall, maintaining bacterial virulence and infectivity. Sortase A cleaves a specific peptide sequence (LPXTG recognition motif) within a target protein between threonine and glycine. The cysteine residue of the active site forms a transient thioacyl intermediate complex with the substrate protein. This intermediate complex and substrate is then immediately attacked by oligo-glycine nucleophiles present on peptide-glycans of the bacterial wall to form an amide bond.
Since Sortase A is a critical enzyme needed to maintain the infectivity of the bacterium, it represents a promising therapeutic target. Especially for the treatment of infections caused by antibiotic-resistant bacteria.
1. Mazmanian, S.K., et al. 1999. "Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall." Science 285(5428): 760-763.
2. Spirig, T., et al.. 2011. "Sortase enzymes in Gram‐positive bacteria." Molecular microbiology 82(5): 1044-1059.