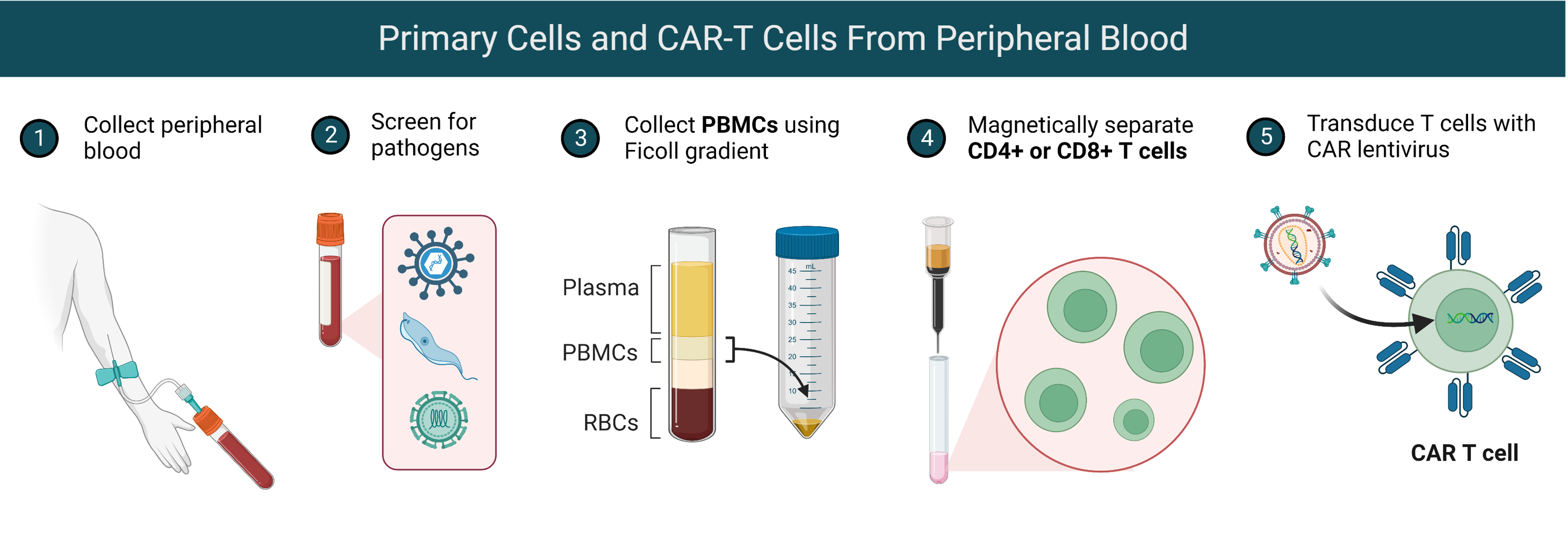
Primary Cells
To support innovation across Immunology and other research areas, BPS Bioscience offers negatively selected, highly enriched CD4+ and CD8+ T cells, and ready-to-use Chimeric Antigen Receptor (CAR)-T cells such as anti-CD19 CAR, anti-BCMA CAR, and control T cells. Primary CAR-T cells, produced by high-titer lentiviral transduction of human primary CD4+ and CD8+ T cells, are used as a positive control in CAR-T cytotoxicity assays, in the design and optimization co-culture assays, or in the screening of CAR-T cell regulators. Get your experiments up to speed with our thaw-and-go solutions for research.

To support innovation across Immunology and other research areas, BPS Bioscience offers negatively selected, highly enriched CD4+ and CD8+ T cells, and ready-to-use Chimeric Antigen Receptor (CAR)-T cells such as anti-CD19 CAR, anti-BCMA CAR, and control T cells. Primary CAR-T cells, produced by high-titer lentiviral transduction of human primary CD4+ and CD8+ T cells, are used as a positive control in CAR-T cytotoxicity assays, in the design and optimization co-culture assays, or in the screening of CAR-T cell regulators. Get your experiments up to speed with our thaw-and-go solutions for research.
We also offer cryopreserved vials of primary human peripheral blood mononuclear cells (PBMCs) from healthy donors freshly isolated from whole blood or leukapheresis samples using Ficoll gradient and are confirmed negative for: Hepatitis B (anti-HBc EIA, HBsAg EIA), Hepatitis C (anti-HCV EIA), Human Immunodeficiency Virus (HIV-1/HIV-2 plus O), Human T-Lymphotropic Virus (HTLV-I/II), HIV-1/HCV/HBV, West Nile Virus, and Trypanosoma cruzi.
Note: Testing cannot guarantee that any sample is completely virus-free. These cells should be treated as potentially infectious and appropriate biological safety level 2 precautions should be used.