HSP70 Assay Kit
The HSP70 Assay Kit is a chemiluminescence assay kit designed to measure HSP70 adenosine triphosphate (ATP) hydrolysis activity for screening and profiling applications using ADP-Glo® Assay as a detection reagent. The HSP70 Assay Kit comes in a convenient 96-well format, with enough purified HSP40 and HSP70 proteins, ATP, and HSP70 assay buffer for 100 enzyme reactions.
Need us to run inhibitor screens or profile your compounds against HSP70? Check out our Heat Shock Protein Screening Services.
- ADP-Glo® Assay (Promega, #V6930)
- Dithiothreitol (DTT; 1 M)
- Microplate reader capable of reading luminescence
- Adjustable micropipettor and sterile tips
- 30°C incubator
| Catalog # | Name | Amount | Storage |
| 50287 | HSP70* | 50 µg | -80°C |
| 78415 | 5x HSP70 Buffer | 1.5 ml | -20°C |
| 79686 | ATP (500 µM) | 100 µl | -20°C |
| 50285 | HSP40* | 50 µg | -80°C |
| 79696 | 96-well plate, white | 1 | Room Temp |
*The concentrations of HSP40 and HSP70 are lot-specific and will be indicated on the tubes containing the proteins.
HSP70 (Heat Shock 70kDa protein) family members are protein chaperones that play an essential role in cellular protein metabolism by acting as polypeptide-binding and release factors. These chaperones ensure the folding of newly synthesized proteins, the formation of dissociation of protein complexes, and protect against stress. HSP40 co-chaperone proteins regulate complex formation between HSP70 and client proteins. Specifically, HSP40 proteins regulate the ATP-dependent binding of HSP70 to polypeptides through their J-domain. The J-domain of HSP40 is thought to interact with HSP70 at an acidic groove located in the ATPase domain. J-domain–HSP70 complex formation plays a critical role in the regulation of HSP70 ATPase activity.
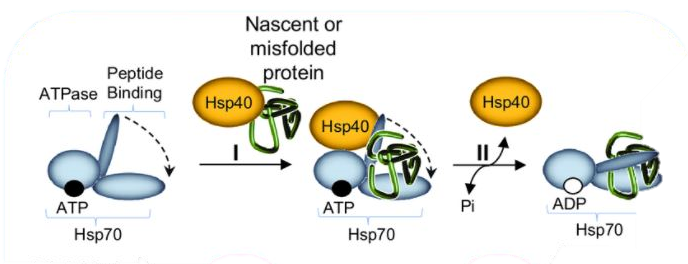
Illustration of the HSP70 machinery reaction cycle. (I) HSP40 mediates the delivery of nascent or misfolded proteins to ATP-bound HSP70; (II) Hydrolysis of ATP to ADP, accelerated by HSP40, results in HSP70 conformational change.
- Fan CY, et al. Mechanisms for regulation of Hsp70 function by Hsp40. Cell stress & chaperones, 2003; 8(4): 309–316.
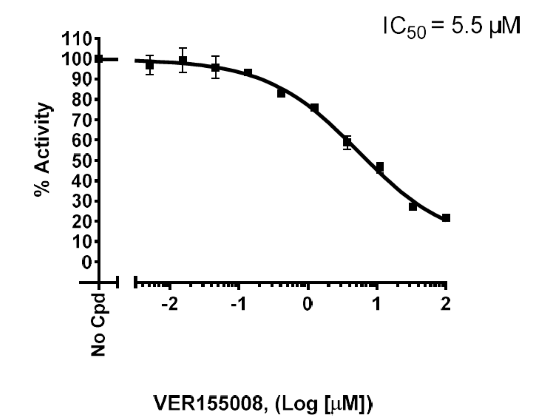
- Massey AJ, et al. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother Pharmacol. 2010; 66(3): 535-45.
- Shiber A, Ravid T. Chaperoning proteins for destruction: diverse roles of Hsp70 chaperones and their cochaperones in targeting misfolded proteins to the proteasome. Biomolecules. 2014; 4(3): 704-24.