Structure and Function of the Claudin Family
Introduction
Claudins (encoded by the multi-gene family CLDN) are tetraspan membrane proteins found in all epithelial and endothelial cells. They serve as crucial structural components of tight junction strands, where the membranes of two adjacent cells connect to form a barrier by self-polymerization and transcellular interactions at the apical-lateral membrane of epithelia. The tight junctions prevent molecules from passing between the cells and maintain cellular polarity. Therefore, claudins play critical roles in the regulation of paracellular permeability, and in the maintenance of cell-cell adhesion in epithelial and endothelial cell sheets (1, 2).
1. Human Claudin (CLDN) Genome
In mammals, the CLDN family consists of 24 genes that exhibit tissue-specific patterns of expression. For example, claudin-7 is highly expressed in small and large intestine while claudin-6 is not observed in this tissue. Humans and primates lack the CLDN-13 gene, which is primarily expressed in mice and rodents (3). Claudin-10, claudin-18, and claudin-19 exist as two isoforms as a result of alternative start sites or splicing, bringing the number of distinct claudin proteins to 27. CLDN genes are small and contain few introns or lack introns altogether. Several pairs of highly homologous CLDN genes are located in proximity of each other on human chromosomes (4). Thus, CLDN6 and CLDN9 are located only 200 base-pair apart on chromosome 16. Similarly, CLDN22 and CLDN24 on chromosome 4, CLDN8 and CLDN17 on chromosome 21, and CLDN3 and CLDN4 on chromosome 7, are all located within 50 kb of each other. This genomic structure suggests that gene duplication has been a driving force in the genetic diversification of claudins. A phylogenetic analysis of the Claudin family, showing the sequence relationships between the genes, revealed how CLDN genes may have evolved (4, 5)
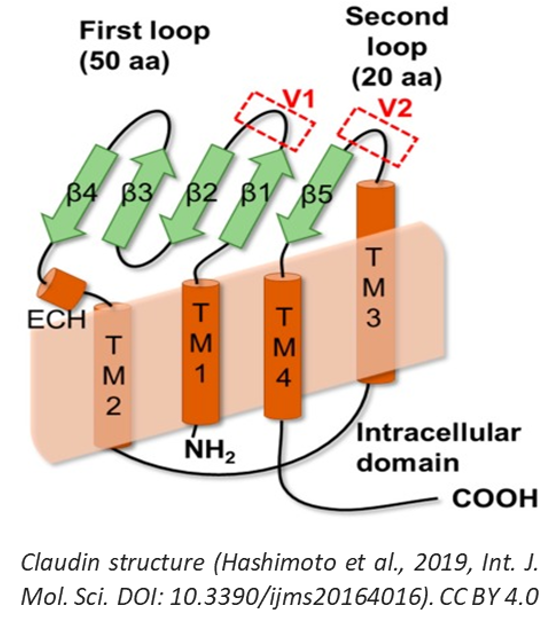
2. Protein Structure
Human claudins are small proteins ranging from 21 to 34 kDa. Their expression is regulated both transcriptionally and post-transcriptionally by various growth factors and cytokines (6). The general structure of a claudin includes four transmembrane domains, with the N- and C-termini extending intracellularly to the cytoplasm, and two extracellular loops termed ECL1 and ECL2.
The N-terminal sequence is usually short, containing approximately 5-7 amino acids, while the C-terminal sequence varies considerably in length and displays the highest sequence heterogeneity. Crystal structures have indicated that the ECLs form an antiparallel β-sheet. ECL1 usually contains four β-strands and a single extracellular helix whereas ECL2 consists of a single β-strand and a cell-surface-exposed domain of the 3d transmembrane span (7, 8). Two highly conserved cysteines residues within ECL1 may form a disulfide bond to stabilize protein conformation. ECL1 is involved in the paracellular pathway and determines the charge selectivity of paracellular transport. Based on molecular modeling studies, it is assumed that ECL2 is folded in a helix-turn-helix motif and is involved in forming dimers with claudins of opposing cell membranes via hydrophobic interactions.
3. Regulation of Claudin Function
Claudins are divided into two groups (classic and non-classic) based on sequence homology and on the number of charged residues in the ECL1 domain (5). Functional analysis indicates that ‘classic' claudins may function as impermeable barriers, reducing the overall paracellular permeability to water and soluble ions. Alternatively, 'non-classic' claudins may constitute pores that facilitate selective ion permeability (5). In addition, claudin family members can interact with each other and with members of the occludin family through their transmembrane domains within a single cell (cis interaction) and through their ECLs between adjacent cells (trans interaction), thereby building strong and tight intercellular junctions with selective permeability to ions and small molecules based on charge and size (9).
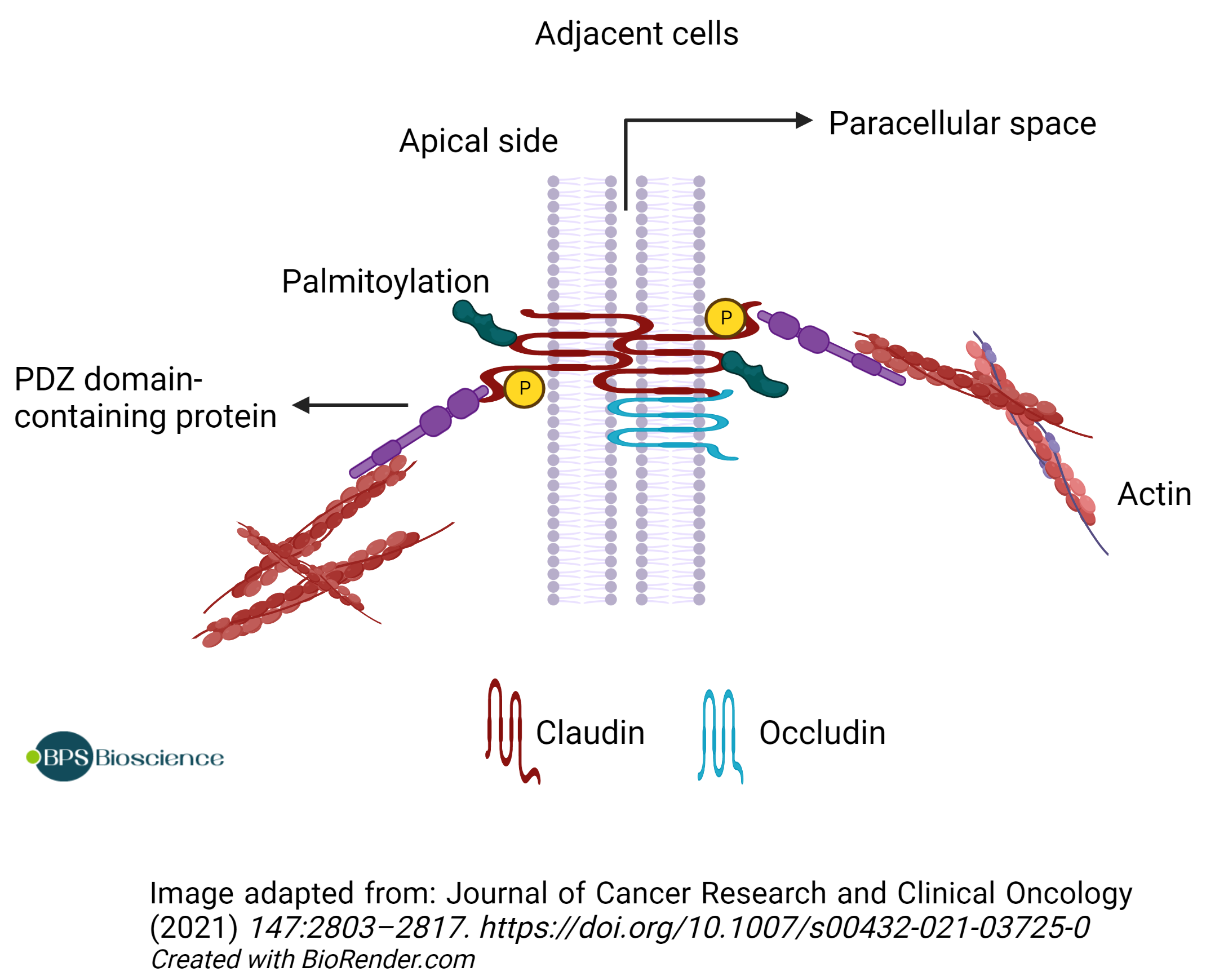
The cytoplasmic C-terminal domain contains a PDZ-domain-binding motif that allows direct interaction with scaffolding proteins containing a PDZ domain. It is also the target of various post-translational modifications such as palmitoylation, which may influence protein stability/half-life, or serine/threonine and tyrosine phosphorylation, which regulates barrier functions (10). For example, phosphorylation of claudin-3 and claudin-4 by protein kinases A and C results in increased paracellular permeability. Similarly, lysine deficient protein kinase 4 (WNK4) can phosphorylate multiple claudins and increase paracellular permeability (11).
4. Claudin Pathophysiology
Several human diseases have been attributed to mutations in CLDN genes. For example, mutations in CLDN1 result in progressive scaling of the skin and obstruction of bile ducts, known as neonatal sclerosing cholangitis with ichthyosis (12). CLDN16 expression is restricted to tight junctions of the thick ascending loop of Henle in the kidney, where magnesium and calcium are reabsorbed paracellularly. Mutations in CLDN16 cause a rare magnesium wasting disorder known as familial hypomagnesemia (13). CLDN19 mutations are associated with ocular conditions, such as macular colobomata, nystagmus, and myopia. CLDN14 is expressed along the endo-cochlear epithelium and when mutated, causes non-syndromic recessive deafness (14). Select claudin family members are also involved in the pathology of infection: claudin-1, claudin-6, and claudin-9 interact with viral proteins and are co-receptors for hepatitis C viral entry (15), whereas claudin-3 and claudin-4 are cell surface receptors for the Clostridium perfringens enterotoxin in the gut (16).
Finally, many members of the claudin family are aberrantly expressed in cancer. Claudin-1 is downregulated in breast and colon cancer, whereas claudin-3 and 4 are highly upregulated in breast, prostate, ovarian, and pancreatic cancer (reviewed in 17). The tight junction has a vital role in maintaining epithelial integrity, and loss of cohesion of the tight junction structure can lead to invasion and ultimately to the metastasis of cancer cells. Hence, aberrant expression of claudins may result in the disorganization of the tight junctions, contributing to EMT (epithelial-mesenchymal transition) with consequences on cell migration and metastasis (10). Alternatively, alteration of expression levels may result in changes in paracellular permeability with consequences on tissue physiology and metabolism. These findings are consistent with the fact that tight junctions are disassembled during tumorigenesis, and claudins are often associated with the promotion of tumor proliferation and invasiveness via the activation of intracellular signaling cascades (10).
Overall, claudin expression may be an indicator of epithelial tissue health and integrity; various studies have studied claudin expression as a biomarker. Attempts at targeting tissue-specific claudins using toxins or toxin fragments such as a C-terminal portion of CPE (Clostridium perfringens enterotoxin) are ongoing.
5. Claudin as Therapeutic Targets
Targeted therapies against claudin have gained a lot of traction, especially in oncology. Claudins are expressed not only in malignant tissues but also in non-malignant tissues. However, most claudins in non-malignant tissues are localized within tight junction and are not accessible to antibodies. The malignant transformation of epithelial tissue causes morphological alterations and impaired cell-to-cell connections that expose claudin proteins to the exterior milieu. This differential exposure between normal and tumor tissue makes claudins near-ideal targets for antibody-based therapeutics, since on-target side effects affecting healthy tissue are less likely to occur.
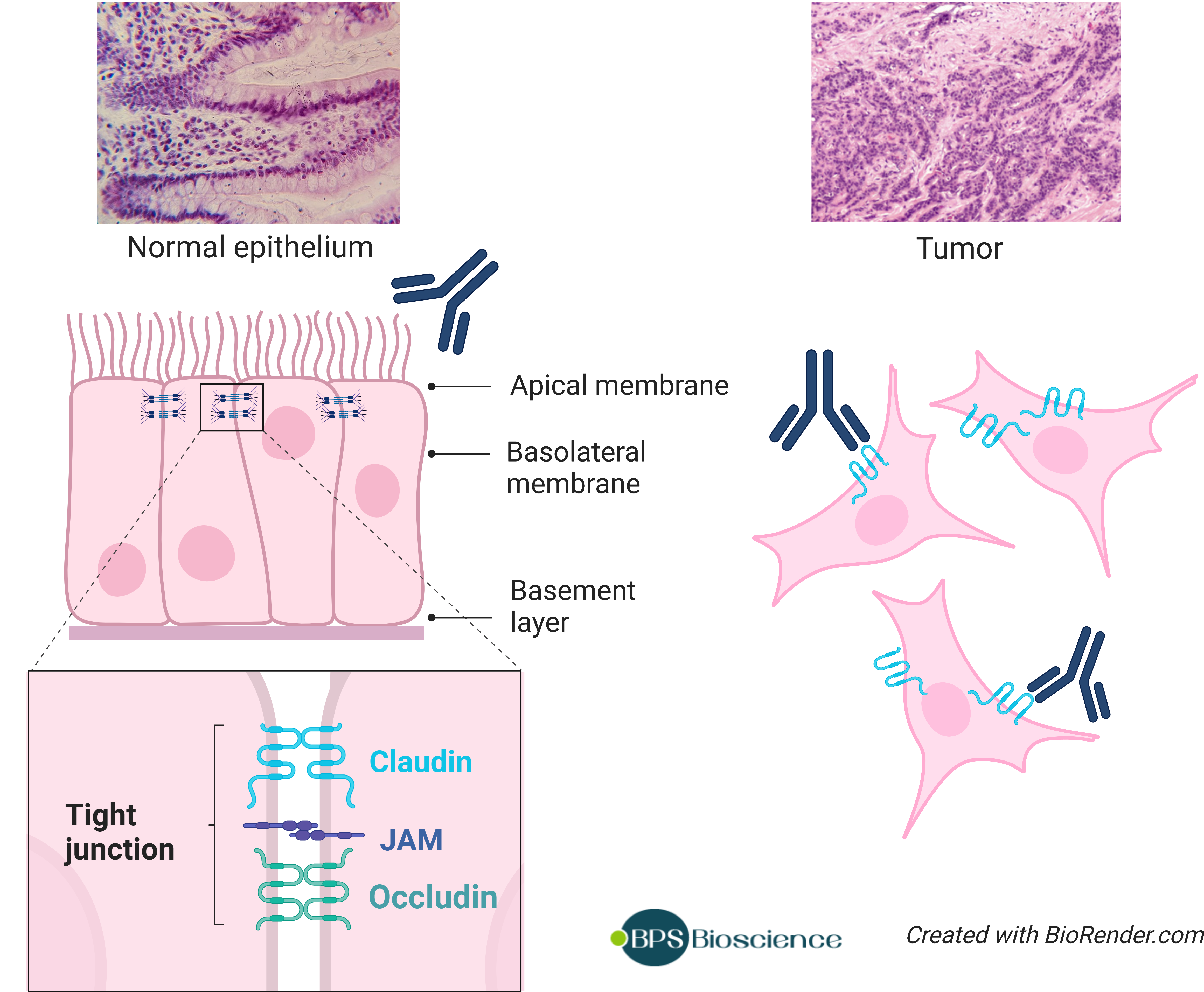
Various drug candidates in development to target claudin proteins:
- The C-terminal domain of Clostridium perfringens enterotoxin (C-CPE), for which several claudins are cell surface receptors, has been proposed as a potential anti-cancer agent for treating pancreatic cancer overexpressing claudin-4 (7).
- Monoclonal antibodies generated for claudin-targeted therapy have shown promise in initial oncology clinical trials (5). Antibodies bind to target proteins with high affinity and high specificity, so long as the protein is accessible. Antibodies against the claudin family are of potential interest in oncology, regenerative medicine, inflammatory diseases, and viral infections (18).
The first anti-claudin antibody to reach the clinic, zolbetuximab (IMAB362), was directed against claudin-18 isoform 2. It demonstrated efficacy in clinical trials for gastric and gastroesophageal junction (GEJ) cancer, paving the way for further development of anti-claudin antibodies.
Conclusion
Interest in the Claudin family as a therapeutic target has been growing steadily. To support research in the field, BPS Bioscience has developed several claudin-expressing cell lines that are ideal for screening anti-claudin antibodies or CAR (Chimeric Antigen Receptor) constructs in a biological context. Anti-claudin antibodies can be used as positive controls in binding or neutralizing assays.
References
(1) Günzel D, Yu ASL. Claudins and the Modulation of Tight Junction Permeability. Physiol Rev. (2013) 93: 525–569. PMID: 23589827
(2) Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol. (20080 295(4): F867-876. PMID: 18480174
(3) Thompson PD, et al. Claudin 13, a Member of the Claudin Family Regulated in Mouse Stress Induced Erythropoiesis. PLoS ONE (2010) 5(9): e12667. PMID: 20844758
(4) Lal-Nag M, Morin PJ. The claudins. Genome Biol (2009) 10: 235. PMID: 19706201
(5) Baltzegar DA, et al. Phylogenetic revision of the claudin gene family. Mar Genomics. (2013) 11: 17-26. PMID: 23726886
(6) González-Mariscal L, et al. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. (2008) 1778(3): 729-756. PMID: 17950242
(7) Hashimoto Y, et al. Potential for Tight Junction Protein-Directed Drug Development Using Claudin Binders and Angubindin-1. Int J Mol Sci. (2019) 20(16): 4016. PMID: 31426497
(8) Suzuki H, et al. Model for the architecture of claudin-based paracellular ion channels through tight junctions. J Mol Biol. (2015) 427(2): 291-297. PMID: 25451028
(9) Furuse M, et al. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. (1999) 147(4): 891-903. PMID: 10562289
(10) Bhat AA, et al. Tight Junction Proteins and Signaling Pathways in Cancer and Inflammation: A Functional Crosstalk. Front Physiol. (2019) 9: 1942. PMID: 30728783
(11) Yamauchi K, et al. Disease-causing mutant WNK4 increases paracellular chloride permeability and phosphorylates claudins. Proc Natl Acad Sci USA. (2004) 101(13): 4690-4694. PMID: 15070779
(12) Hadj-Rabia S, et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology. (2004) 127: 1386-1390. Erratum in: Gastroenterology. 2005 Feb;128(2):524. PMID: 15521008
(13) Simon DB, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. (1999) 285(5424): 103-106. PMID: 10390358
(14) Wilcox ER, et al. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell. (2001) 104(1): 165-172. PMID: 11163249
(15) Evans MJ, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. (2007) 446(7137): 801-805. PMID: 17325668
(16) Katahira J, et al. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chem. (1997) 272(42): 26652-26658. PMID: 9334247
(17) Li J. Dysregulated expression of claudins in cancer. Oncol Lett. (2021) 22(3): 641. PMID: 34386063
(18) Hashimoto Y, et al. Claudin-Directed Drug Development. J. Pharm. Exp. Ther. (2019) 368 (2): 179-186. PMID: 30530622

