Cholesterol & the PCSK9 Pathway
Role & Significance
Cholesterol is an important molecule for normal cell function, and serves as a precursor for steroid hormones and bile acids. Reducing LDL-C levels, most commonly by statins, has been shown to substantially reduce CVD events. However, statin therapy alone is not adequate to lower LDL-C for many patients, especially those with familial hypercholesterolemia.
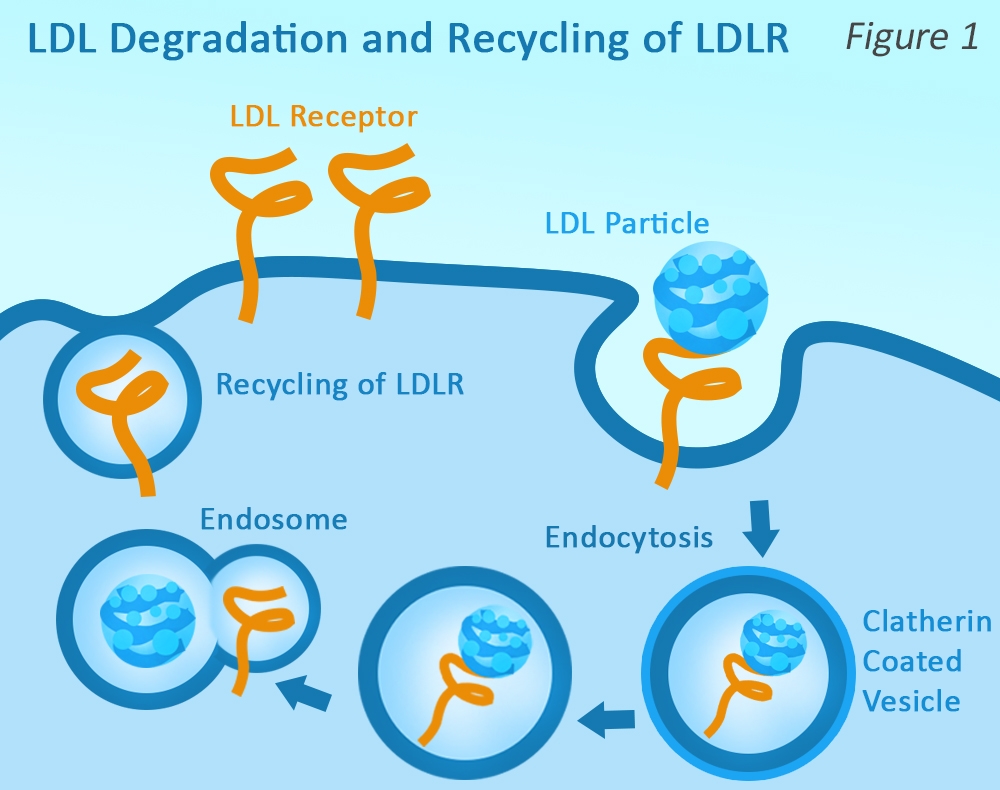
LDL receptors (LDLRs) play a key role in regulating LDL-C levels in plasma by influencing the clearance of LDL-C from circulation. LDLRs bind to LDL, and the complexes enter the cell by endocytosis. Upon exposure to the acidic pH of the endosome, the LDL is released, allowing LDLR recycling to the membrane.
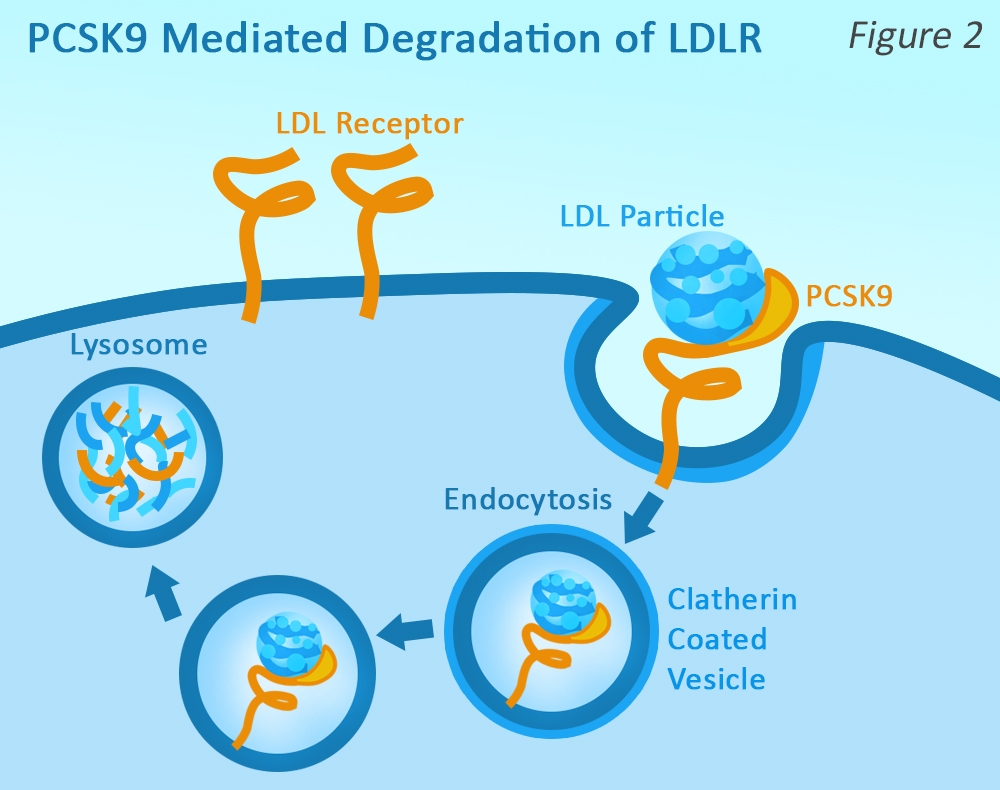
PCSK9 is synthesized expressed in the liver, but is secreted into the plasma. Plasma PCSK9 binds to the extracellular region of LDLR (at a separate location than the ApoB100 binding site), and when the LDLR is internalized, it is again transported to the endosome.
However, the PCSK9 causes a conformational change in the LDLR, marking it for lysosomal degradation, and preventing it from being recycled back to the cell surface. Therefore, PCSK9 reduces the amount of LDLR that is available to remove LDL-C from circulation, resulting in higher levels of plasma LDL-C.
PCSK9 Inhibitors
Inhibitors of proprotein convertase subtilisin/kexin type 9 (PCSK9) have emerged as a novel therapeutic class that reduce LDL-C through increased hepatic clearance. In 2015, the US Food and Drug Administration (FDA) approved PCSK9 inhibitors for patients on maximally tolerated statin therapy who “require additional lowering of LDL” and as an effective alternate therapy in patients intolerant of statins1. Monoclonal antibodies against PCSK9 have been shown to lower plasma LDL-C levels by ~60%, even in patients already receiving maximum-dose statin therapy.2 PCSK9 inhibition has also shown great promise for preventing future heart attacks and strokes in patients with established CVD.
Because CVD is responsible for so many deaths worldwide, PCSK9 inhibitor therapies had one of the shortest durations from discovery to development and approval. Two PCSK9 inhibitors have received FDA approval: alirocumab (Praluent), from Regeneron and evolocumab (Repatha), from Amgen. Not surprisingly, Novartis, Eli Lilly and Pfizer all have monoclonal PCSK9 inhibitors in late phase clinical trials. Despite the success of anti-PCSK9 monoclonals, the cost of treatment remains prohibitive for some patients. Amgen recently promised to reduce the annual cost of its Repatha for US patients from $14,100 to $5850 by 20203, but this is still very expensive.
Additional Directions
A number of pharma companies have focused on other strategies for PCSK9 inhibition, including prevention of the formation of PCSK9 by antisense oligonucleotides or siRNA (Anmylam), or by preventing the binding of mature PCSK9 to LDLR via small molecule inhibitors (Shifa Biomedical), adnectin (BMS-Adnexux), or mimetic peptides (Merck, Seronexus)4. Researchers are also studying various combination therapies and uncovering other PCSK9 functions, such as lowering the plasma concentrations of lipoprotein(a)5.
References
- Kosmas, C.E., et al. Drugs Context. 2017; 6: 212511.
- Sabatine, M.S. 2019. Nature Reviews Cardiology 16:155–165.
- https://www.ajmc.com/newsroom/amgen-announces-60-reduction-in-list-price-of-pcsk9-inhibitor-evolocumab
- Moumita, G.L., and Laskar, A. 2015. IJEPP 1 (1): 29-36.
- Romagnuolo R, et al. 2015. J Biol Chem. 290(18):11649-62.
Antibodies
Assay Kit
- PCSK9-LDLR TR-FRET Assay Kit
- PCSK9(D374T)-LDLR TR-FRET Assay Kit
- PCSK9 [Biotinylated] - LDLR Binding Assay Kit
Proteins
- PCSK9, C-terminal His-Avi-tag, Biotin-labeled
- PCSK9, His-Avi-Tag (Mouse)
- PCSK9, His-Avi-Tag (Rat) (20 µg, 50 µg)
- PCSK9, His-tag ( 20 µg, 50 µg)
- PCSK9(D374T), Biotin-labeled
- LDLR, Biotin-labeled
- LDLR, FLAG-tag
- LDLR, FLAG-Tag, Eu-labeled
- LDLR, FLAG-Avi-Tag (Mouse)

